Chapter 22 ~ Pesticides
Key Concepts
After completing this chapter, you will be able to:
- Explain the notions of “pest” and “weed,” and provide reasons why it may be necessary to decrease their abundance.
- Differentiate pesticides by the pests to which they are targeted.
- Classify pesticides into major chemical groups.
- Outline the risks and benefits of pesticide use in sanitation, agriculture, forestry, and horticulture.
- Explain why there is a global contamination of organisms with DDT and related organochlorines, and describe the associated ecological damage.
- Outline the ecological damage caused by carbofuran, and explain why it took so long for the use of this insecticide to be banned.
- Describe the economic benefits and ecological risks of pesticide use in forestry.
- Outline the concept of integrated pest management, and explain whether it is applicable to all pest-management problems.
Introduction
Humans are constantly engaged in struggles against competitors and diseases. One way to gain an advantage in many of those ecological interactions is through the use of pesticides. These substances are used to protect crop plants, livestock, domestic animals, and people from damage and diseases caused by microorganisms, fungi, insects, rodents, and other “pests,” and to defend crops from competition with unwanted but abundant “weeds.”
It is important to understand that the use of words like “pest” and “weed” is highly contextual. In most situations, for example, white-tailed deer are valued for their wild beauty, and they provide economic and subsistence benefits through hunting. However, this animal may also be considered a pest when it feeds in a garden, agricultural field, or forestry plantation. The same is true, to some degree, of other species that are considered to be a pest or weed.
People have been using pesticides for a long time (Hayes, 1991). There are records of unspecified chemicals being used by Egyptians to drive fleas from their home as long as 3,500 years ago. Arsenic has been used as an insecticide in China for at least 2,900 years. In his epic poem the Odyssey, the Greek poet Homer (writing about 2,800 years ago) referred to the burning of sulphur (which generates toxic SO2 gas) to purge homes of vermin such as fleas.
However, pesticide use has become much more common in modern times, and an enormously wider variety of substances is being used. At least 300 insecticides, 290 herbicides, 165 fungicides, and many other pesticidal chemicals are available in more than 3,000 different formulations. Strictly speaking, a pesticide is a product that consists of a formulation of several chemicals – the “active ingredient” attacks the pest, while various “inert ingredients” enhance its effectiveness (see In Detail 22.1). Even larger numbers of “commercial products” are available, because many involve similar formulations manufactured by different companies.
Almost all pesticides are chemicals. The active ingredients of some of them are based on natural biochemicals that are extracted from plants grown for that purpose, while others are inorganic chemicals based on toxic metals or compounds of arsenic. Most modern pesticides, however, are organic chemicals that have been synthesized by chemists. The costs of developing a new pesticide and testing it for its efficacy (effectiveness against pests), toxicological properties, and environmental effects are quite large, equivalent to tens of millions of dollars per chemical. However, if an effective pesticide against an important pest is discovered, the profits are potentially huge, and therefore industry willingly pays the high development costs.
People have acquired important benefits from many uses of pesticides:
- increased yields of crops, because of protection from diseases, competition, defoliation, and parasites
- revention of much spoilage and destruction of stored food
- avoidance of certain diseases, thereby conserving health and saving the lives of millions of people and domestic animals
This is not to say, however, that more pesticide use would achieve even better results. In fact, it has been argued that pesticide use in North America could be decreased by half without greatly affecting crop yields (Pimentel et al., 1991). The European Union (EU) has taken some forceful steps to reduce pesticide use within its jurisdiction (Pesticide News, 2003). In 2003, EU permits for 320 pesticides were revoked, and as many as another 180 were scheduled for delisting in 2010; in total, half of the pesticides used in 1993 were no longer permitted in 2010. In large part, the withdrawals involve obsolete pesticides and others of minor commercial importance, for which the owners do not want to invest the large amounts of money needed to assure EU regulators that their products are safe for people and the environment. Similar actions are also occurring in North America, but they are less advanced than in the EU. Because of the substantial benefits that can be derived from the use of pesticides, their consumption has increased enormously during the past half-century. Overall, the use of pesticides increased ten-fold in North America between 1945 and 1989 (Pimentel et al., 1992), although it has since levelled off. Pesticide use is now a firmly integrated component of technological systems that are widely used in modern agriculture, forestry, horticulture, and public health management in most parts of the world.
Unfortunately, the considerable benefits of pesticides are partially offset by damage their use causes to ecosystems and sometimes to human health. Each year, about one-million people are poisoned by pesticides, with as many as 20-thousand fatalities (Pimentel et al., 1992). Although developing countries account for only about 20% of global pesticide use, they sustain about half of the poisonings. This is because relatively toxic insecticides are used in many developing countries, by a workforce whose widespread illiteracy hinders the understanding of instructions for proper use, and whose safety is further compromised by poor enforcement of regulations and by inadequate use of protective equipment and clothing.
The most tragic case of pesticide-related poisoning occurred in 1984 at Bhopal, India. About 2,800 people were killed and 20-thousand seriously poisoned when a factory accidentally released 40 tonnes of methyl isocyanate vapour to the atmosphere. Methyl isocyanate is a precursor chemical of carbamate insecticides (Rozencranz, 1988).
In addition, many pesticide applications cause ecological damage by killing non-target organisms (organisms that are not considered to be a pest). This damage is particularly important for broad-spectrum pesticides, which are toxic to organisms in addition to the specific pest. Pesticides applied as a broadcast spray are spread over a large area, such as an agricultural field, lawn, or stand of forest.
If a broad-spectrum pesticide is broadcast-sprayed, many non-target organisms are exposed and they may be damaged or killed. For example, in a typical agricultural field or forestry plantation, only a few plant species are abundant enough to compete significantly with crops and reduce their productivity. These are the “weeds” that are the target of a broadcast herbicide application, but many other plants are also affected. The non-target plants may provide habitat or food for animals, and they help to prevent erosion and loss of nutrients. These benefits are degraded by non-target damage – by damage to organisms that are not pests. Similarly, broadcast insecticide spraying causes non-target mortality to many beneficial arthropods in addition to the species that is considered to be a pest. Many birds, mammals, and other creatures may also be poisoned. The non-target mortality may include predators and competitors of the pest, an ecological change that may release it from some of its biological controls. Clearly, the great challenge of pest control is to develop effective, pest-specific pesticides and to invent non-pesticidal methods.
Pesticide use has been expanding rapidly, and this is happening in all countries, although to varying degrees. Although much is known about the environmental damage caused by the use of pesticides, not all of the potential effects are well understood. In this chapter we examine the nature of pesticides and their important uses. We then examine cases of ecological damage caused by their routine use to deal with pest-management problems.
In Detail 22.1. Pesticides, Formulations, and Inert Ingredients. A commercial pesticide product is a mixture of chemicals that can be used to kill or otherwise control pests. The “active ingredient” is the chemical that actually attacks the pest, while so-called “inert ingredients” are added to the formulation to enhance its effectiveness. Inert ingredients may do this by making the pesticide easier to apply (such as by making it soluble in water), by helping it to spread or stick to leaf surfaces, or by stabilizing the formulation to increase its shelf-life.
Many inert ingredients are, however, biologically active, so they are not really passive substances (Environment Canada, 2001; EPA, 2005). It is more realistic to refer to them as “other ingredients.” In general, the percentage of other ingredients in a pesticide is specified on the product label, but their identity and concentrations are not given because they are considered to be proprietary information of commercial value. Sometimes, however, a manufacturer will identify these ingredients, and may even specify their concentration. Some inert ingredients carry risks of causing toxicity through normal use of the pesticide. Examples of particular concern include chlorobenzene, dioctyl phthalate, formaldehyde, hexane, hydroquinone, isophorone, nonylphenol, phenol, and rhodamine.
One inert ingredient that has engendered particular controversy about its potential toxicity to humans is nonylphenol (NP), which is used as an emulsifier in some pesticides. NP is a degradation product of nonylphenol ethoxylates (NPEs), which have been used for decades as detergents and emulsifiers. They are used in manufacturing processes for paint, paper, pesticides, petrochemicals, resins, steel, and textiles, and are ingredients in many cleaning products.
NPEs and NP are anthropogenic chemicals that enter the environment with discharges of industrial and municipal wastewater. NPEs degrade by microbial reactions, and some of the metabolites are bioactive through toxicity and estrogenic (hormonal) effects, including NP, nonylphenol diethoxylate, nonylphenol ethoxylate, nonylphenoxyacetic acid, and nonylphenoxyethoxyacetic acid. These may have a moderate persistence in the environment, especially in anaerobic habitats and in groundwater, and they now have developed a widespread but low level of contamination and bioaccumulation. Species vary widely in their vulnerability to toxicity from NP and NPEs, but many studies have reported toxic and estrogenic effects on aquatic organisms.
Some toxicologists believe that humans are also exposed to significant risks from these chemicals, through the use of consumer products, food, and other pathways. In a risk assessment, Environment Canada (2001) concluded that “nonylphenol and its ethoxylates are entering the environment in a quantity or concentration . . . [that has] or may have an immediate or long-term harmful effect on the environment or its biological diversity,” so they should be regulated as “toxic” chemicals under the Canadian Environmental Protection Act. Although these chemicals are not “considered a priority for investigation of options to reduce human exposure through control of sources,” it was recommended that further studies of their bioactivity and environmental risks be undertaken.
Although the major releases of NP and related chemicals are via industrial and municipal effluents, they are also present as “other ingredients” in pesticides. This has led to controversy about damage that may be caused to people and wild organisms exposed to NPEs and NP through the use of pesticides. The case of NPEs and their metabolites reinforces the fact that product formulations should be known and comprehensively evaluated when considering the risks of pesticide use to human and environmental safety.
The Nature of Pesticides
Classification by Target
Pesticides are defined by their usefulness in killing or otherwise decreasing the abundance of species that are deemed to be “pests.” Pesticides are, however, an extremely diverse group of substances. To better understand their usefulness and toxicity, and the damage they cause, it is helpful to categorize them in various ways. One classification is based on the intended target of the use:
- a fungicide is used against fungi that cause diseases and other damage to crop plants and animals
- a herbicide is used to kill weeds, which are unwanted plants that interfere with some human purpose; most use in agriculture and forestry is intended to release crop plants from competition, while horticultural use is mostly for aesthetics
- an insecticide is used to kill insects that are pests in agriculture, horticulture, or forestry, or that spread diseases such as mosquito vectors (a path by which a disease is spread) of malaria, yellow fever, and encephalitis
- an acaricide is used to kill mites that are pests in agriculture, and ticks that are vectors of ailments such as Lyme disease and typhus
- a molluscicide is used to kill snails and slugs in agriculture and gardens, and aquatic snails that are vectors of diseases such as schistosomiasis
- a nematicide is used against nematodes, which can damage the roots of agricultural plants
- a rodenticide is used to control mice, rats, gophers, and other rodents that are pests in agriculture or around the home
- an avicide is used to kill birds, which are sometimes considered pests in agriculture
- a piscicide is used to kill fish, which may be pests in aquaculture
- an algicide is used to kill unwanted growths of algae, for example, in swimming pools
- bactericides, disinfectants, and antibiotics are used to control infections and diseases caused by bacteria (Note that antibiotics are not actually classified as “pesticides” under the Pest Control Products Act)
Chemical Classification
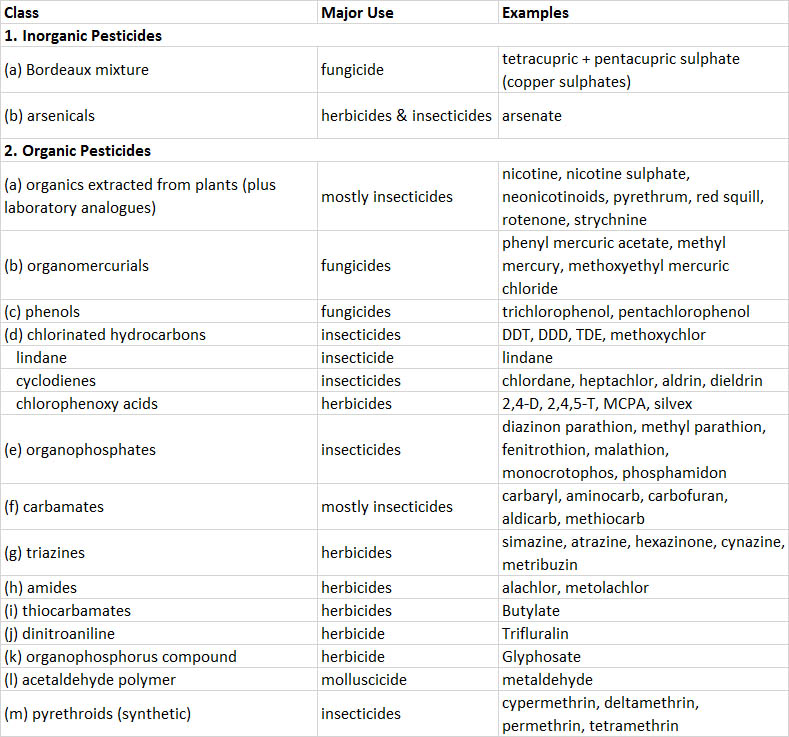
Because almost all pesticides are chemicals, they can be categorized according to similarities in chemical structure. The most important groups are described below and in Table 22.1. A few “non-chemical” pesticides are based on microbes, and are discussed later under “Biological Pesticides.”
Table 22.1. Some Important Pesticides.
Inorganic pesticides are compounds that contain toxic elements such as arsenic, copper, lead, or mercury. They are highly persistent in terrestrial environments, being only slowly dispersed by leaching and erosion by wind or water. Recently, inorganic pesticides have been widely replaced by synthetic organics. Prominent examples include Bordeaux mixture, a complex of copper-based compounds that is used as a fungicide to protect fruit and vegetable crops, and arsenicals such as arsenic trioxide, sodium arsenite, and calcium arsenate, which are used as herbicides and soil sterilants. Paris green, lead arsenate, and calcium arsenate are used as insecticides.
Organic pesticides are mostly synthesized chemicals, but some are natural toxins produced by certain plants that are extracted and used as pesticides. Important examples include the following:
- Natural organic pesticides are extracted from plants. For example, nicotine and related alkaloids are extracted from tobacco (Nicotiana tabacum) and used as insecticides, usually applied as nicotine sulphate. Neonicotinoids are a synthetic analogue. Pyrethrum is a complex of chemicals extracted from certain chrysanthemums (Chrysanthemum cinerariaefolium and C. coccinium) and used as an insecticide. Rotenone is extracted from several tropical shrubs (Derris elliptica and Lonchocarpus utilis) and used as an insecticide, rodenticide, or piscicide. Red squill, extracted from the sea onion (Scilla maritima), is a rodenticide, as is strychnine, extracted from the tropical shrub Strychnos nux-vomica.
- Synthetic organometallic pesticides are used as fungicides and include organomercurials such as methylmercury and phenylmercuric acetate.
- Phenols include trichlorophenols, tetrachlorophenol, and pentachlorophenol, which are fungicides used mostly to preserve wood.
- Chlorinated hydrocarbons (organochlorines) are a diverse group of synthetic pesticides (see In Detail 22.2). Most are quite persistent, having a half-life of about 10 years in soil because they are not easily degraded by microorganisms or by physical agents such as sunlight or heat. The persistence of organochlorines, coupled with their strongly lipophilic nature (they are highly soluble in fats and lipids, but virtually insoluble in water), means that they strongly bioconcentrate and biomagnify, with the highest concentrations occurring in top predators (see In Detail 18.1 and Figure 22.1). Organochlorines include the following:
- DDT and its insecticidal relatives, such as DDD and methoxychlor, were once widely used insecticides. Because of bans in North America and Europe in the early 1970s, their use is now confined to tropical countries. DDE is a persistent non-insecticidal metabolite of DDT and DDD that accumulates in organisms.
- Lindane is the active constituent of hexachlorocyclohexane, an insecticide.
- Cyclodienes are highly chlorinated cyclic hydrocarbons, such as chlordane, heptachlor, aldrin, and dieldrin, all of which are insecticides.
- Chlorophenoxy acids have growth-regulating influences on plants and are used as herbicides against broad-leafed weeds. The most important compound is 2,4-D, but others are 2,4,5-T, MCPA, and silvex.
- Other organochlorines include polychlorinated biphenyls (PCBs), dioxins, and furans. These are not pesticides but are mentioned here because their ecotoxicological properties are similar to those of the pesticide organochlorines: they are persistent in the environment and are lipophilic, so they bioconcentrate and food-web magnify.
- Organophosphorus pesticides are used mostly as insecticides, acaricides, and nematicides. They are not persistent in the environment, but are extremely toxic to arthropods and also to non-target fish, birds, and mammals. Parathion, fenitrothion, malathion, and phosphamidon are prominent examples of organophosphate insecticides. Glyphosate, a phosphonoalkyl compound, is an important herbicide (it is not toxic to animals).
- Carbamate pesticides have a moderate persistence in the environment but are highly toxic to arthropods, and in some cases, to vertebrates. Aminocarb, carbaryl, and carbofuran are important insecticides.
- Triazine pesticides are used as herbicides and sometimes as soil sterilants. Prominent examples are atrazine, simazine, and hexazinone.
- Synthetic pyrethroids are analogues of natural pyrethrum and are used mostly as insecticides and acaricides. Pyrethroids are highly toxic to invertebrates and fish, but they are of variable toxicity to mammals and of low toxicity to birds. Important examples are cypermethrin, deltamethrin, permethrin, synthetic pyrethrum and pyrethrins, and tetramethrin.
- Biological Pesticides are formulations of microbes that are pathogenic to specific pests and so have a narrow spectrum of toxicity in ecosystems. The best examples are insecticides based on the bacterium Bacillus thuringiensis (B.t.), types of which are used against moths, flies, and beetles. Insecticides based on nuclear polyhedrosis virus (NPV) and insect hormones have also been developed.
- Genetically modified organisms (GMOs; see Environmental Issues 6.1) have been biologically “engineered” by inserting portions of DNA from another species into their genome. This high-tech procedure has been used to develop new varieties of commercial crops that are more resistant to certain pesticides or pests, which can make it easier to cultivate them. For example, GMO varieties of soybean and canola have been developed to be resistant to glyphosate, meaning this herbicide can be used on those crops, providing reduced costs of energy and machinery to control weeds. In addition, there are GMO varieties of maize (corn) that contain DNA of Bacillus thuringiensis, which provides resistance to some insect pests and allows farmers to use less insecticide. These and other GMO crops are widely cultivated in North America, although they are banned in many countries, including most of Europe and Brazil. The use of these GMO crops is controversial because there is incomplete knowledge about the biological and ecological risks of their use, including the potential escape of their GMO factors to wild plants.
Figure 22.1. Residues of PCBs in the Food Web of Lake Ontario. Organochlorine insecticides, such as DDT, DDD, and dieldrin, show a similar pattern of bioaccumulation and biomagnification as PCBs, but their residue levels are different. Source: Data from Environmental Protection Agency (2003).
In Detail 22.2. Chemical Structure of Organochlorines Organochlorines are a diverse group of compounds that are made up of atoms of carbon, hydrogen, and chlorine. Their biochemical activity (including toxicity) and potential usefulness depend entirely on their chemical structure. Certain organochlorines are used as insecticides (such as DDT, DDD, dieldrin), herbicides (2,4-D, 2,4,5-T), or insulating fluids (PCBs). Others have no particular use at all but are nevertheless important environmental contaminants. For example, DDT and DDD are metabolized in organisms to DDE, which is a non-pesticide that can accumulate to a high concentration in fatty tissues. Another example is the extremely toxic dioxin TCDD, which is non-intentionally synthesized as a contaminant during the manufacturing of certain organochlorines (such as trichlorophenol) and through reactions occurring when organic waste is incinerated.
The following diagrams illustrate the specific chemical structures of a number of environmentally important organochlorines. In the diagrams, the ring-like structures are derived from benzene, which has the formula C6H6. Organochlorines are formed by the substitution of one or more of the hydrogen atoms by chlorine atoms. Note the following:
- There is great similarity among DDT, DDD, and DDE, which differ by only a single chlorine atom
- Similarly, 2,4-D and 2,4,5-T differ by only one chlorine atom
- PCBs are a complex mixture of molecules with a basic biphenyl structure, but varying in the amount of substitution of chlorine for hydrogen atoms; in the diagram, “X” can be either H or Cl
- TCDD, strictly speaking, is not an organochlorine because it contains two oxygen atoms
DDT or 2,2-bis-(p-chlorophenyl)-1,1,1-trichloroethane (an insecticide)
DDD or 2,2-bis-(p-chlorophenyl)-1,1-dichloroethane (an insecticide)
DDE or 2,2-bis-(p-chlorophenyl)-1,1-dichloroethylene (non-insecticidal metabolite of DDT and DDD)
2,4-D or 2,4-dichlorophenoxyacetic acid (a herbicide)
2,4,5-T or 2,4,5-trichlorophenoxyacetic acid (a herbicide)
PCBs or polychlorinated biphenyls (electrical insulating fluid)
TCDD or 2,3,7,8-tetrachlorodibenzo-p-dioxin (a trace contaminant)
Pesticide Use
The global use of pesticides was about 2.4-million tonnes in 2007, a total that includes insecticides, herbicides, fungicides, preservatives, and disinfectants (Grube et al., 2007). Global pesticide trade in 2007 had a value of about US$39 billion. About 39% (by weight) of pesticides used were herbicides; insecticides accounted for 18%, fungicides for 10%, and “other chemicals” for 33% (mostly used as soil fumigants). Data for the United States were herbicides 44%, insecticides 9%, and fungicides 6%. Data for Canada are not available but would be proportionately similar to those for the United States (Canada accounts for about one-ninth of the North American market for pesticides). Total expenditures for pesticides in the United States were about US$12 billion in 2007. The following is a top-20 list of conventional pesticides recently used in the United States (values are 106 kg of active ingredient per year):
- glyphosate; herbicide; 83
- atrazine; herbicide; 34
- metam sodium; soil fumigant; 24
- metolachlor-S; herbicide; 15
- acetochlor; herbicide; 14
- dichloropropene; fumigant; 13
- 2,4-D; herbicide; 12
- methyl bromide; soil fumigant; 6
- chloropicrin; fumigant; 5
- pendimethalin; herbicide; 4
- ethephon; plant growth regulator; 4
- chlorothalonil; fumigant; 4
- metam potassium; fumigant; 4
- chloropyrifos; insecticide; 4
- copper hydroxoide; fumigant; 4
- simazine; herbicide; 3
- trifluralin; herbicide; 3
- propanil; herbicide; 3
- mancozeb; fungicide; 3
- aldicarb; insecticide; 3
In addition, about 1,180 × 106 kg of chlorine and hypochlorite was used as disinfecting agents, 35 × 106kg of sulphur as fungicide, 47 × 106 kg of oil as insecticide, 22 × 106 kg of sulphuric acid as fumigant, and 434 × 106 kg of various substances as wood preservatives. Strictly speaking, these chemicals are not considered pesticides, even though they are used against certain pests. The most important uses of pesticides are in agriculture and forestry, around the home, and in human health and sanitation programs. We examine these uses in the following sections.
Pesticide Use for Human Health
Various insects and ticks are vectors that transmit pathogens among individuals of the same species, or from an alternate host to people, or to domestic and wild animals. Important human diseases that are vectored by invertebrates incluse the following:
- malaria, which is caused by the protozoan Plasmodium and spread to people by Anopheles mosquitoes
- yellow fever, encephalitis, and West Nile virus, caused by viruses and spread by mosquitoes
- sleeping sickness, caused by the protozoan Trypanosoma and spread by the tsetse fly Glossina
- plague or black death, caused by the bacterium Yersinia pestis and transmitted by the rat flea Xenopsylla cheops
- typhoid, caused by the bacterium Rickettsia prowazeki and transmitted by the louse Pediculus humanus
- schistosomiasis or bilharziasis, caused by the blood fluke Schistosoma, with freshwater snails as the alternate host
To varying degrees, the incidence of these maladies can be controlled by using pesticides against the invertebrate vectors or the alternate hosts. The abundance of mosquitoes, for example, can be reduced by spraying insecticide in their aquatic breeding habitat or by applying a persistent insecticide to the interior walls of buildings, where they rest. Similarly, people infested with body lice may receive a surface dusting with an insecticide – this was an early use of DDT. Plague can be controlled by using rodenticide along with sanitation programs to reduce rat populations. Over the past half-century, pesticides have decreased the abundance of vectors and alternate hosts and have spared hundreds of millions of humans from the debilitating or deadly effects of certain diseases, particularly in tropical countries. (This has been an important factor in reducing death rates and allowed rapid population growth.)
In fact, one of the first important uses of DDT was in Naples, Italy, during the Second World War, to prevent a deadly plague of typhus that could have decimated Allied troops and the civilian population. Because of the success of this use of DDT and its contribution to the victorious war effort, the British prime minister at the time, Winston Churchill, referred to the insecticide as “that miraculous DDT powder.”
Malaria has long been an important disease in tropical countries. During the 1950s, about 5% of the global population was infected with malaria. The use of insecticide to reduce the abundance of mosquitoes achieved huge reductions in the incidence of malaria in some countries. For instance, during 1933-1935, India recorded about 100-million cases of malaria per year and 750-thousand deaths. However, the incidence was reduced to 150-thousand cases and 1,500 deaths in 1966 because of spraying with DDT and the draining of mosquito-breeding wetlands (McEwen and Stephenson, 1979). Similarly, 2.9-million cases of malaria occurred in Sri Lanka in 1934, and 2.8 million in 1946, but DDT use helped to reduce that incidence to only 17 cases in 1963 (Hayes, 1991). However, malaria has recently been resurging in some tropical countries, partly because mosquitoes have developed a genetically based tolerance of previously effective insecticides. Many people are again being exposed to the malarial parasite, although the disease can today be controlled by drugs that prevent Plasmodium from multiplying in the blood. (However, there are also signs that Plasmodium is becoming resistant to those drugs.)
Pesticides and Agriculture
Modern agriculture is a highly technological activity. Machines, energy, fertilizer, pesticides, and high-yield crop varieties are used in intensive management systems to grow crops (see Chapters 14 and 24). The role of pesticides is to help control the abundance of the following problems:
- weeds that compete with crop plants
- invertebrates and rodents that feed on crops or stored produce
- microbial diseases that can kill the crop or diminish its yield
Undeniably, these uses of pesticides are important factors in modern agriculture. Even with pesticide use, the damage caused by pests and diseases around the world are equivalent to about 24% of the potential crop of wheat, 46% of rice, 35% of corn (maize), 55% of sugar cane, 37% of grapes, and 28% of vegetables (McEwen and Stephenson, 1979). In North America pests destroy about 37% of the potential production of food and fibre crops (Pimentel et al., 1992).
Of course, management practices in agriculture have intensified greatly, particularly during the twentieth century and since. This change has resulted in increases in crop productivity. The gains in agricultural yield have been largely achieved by the combined influences of the following:
- fossil-fuelled mechanization
- fertilizer use
- improved crop varieties grown in monocultural systems
- the use of pesticides
The recent intensification of agrotechnology is sometimes referred to as the “green revolution”. Although agricultural yields have increased greatly, it must be recognized that the gains are highly subsidized by the following (see also Chapters 14 and 24):
- intense use (and depletion) of non-renewable fossil fuels and metals
- depletion of potentially renewable resources, such as soil fertility and tilth, and groundwater and surface water needed for irrigation
- loss of soil mass through erosion
- extensive salinization of soils in semi-arid regions (caused by inappropriate methods of irrigation)
- ecological damage associated with the conversion of natural ecosystems into agricultural ones
- ecotoxicological damage caused by the use of pesticides
In the United States, for example, the yield of corn was typically about 1.4 t/ha-yr in 1933, but it increased to 4.2 t/ha-yr in 1963 and to 5.1-7.1 t/ ha-yr during 1978-1984. In Mexico, wheat yields increased from 0.75 t/ ha-yr in 1945 to 2.6 t/ ha-yr in 1964. The yield of rice in Japan increased from a pre-war average of 1.8 t/ ha-yr to 4.0 t/ ha-yr in 1963, while in the United States, rice yields have reached 4.9–5.5 t/ ha-yr (Hayes, 1991). Similar gains in agricultural yield have been realized in Canada (see Figure 14.1).
Almost all intensively managed agricultural systems depend to some degree on pest control. High-yield crop varieties are often vulnerable to infestation by insect pests, diseases, and competition from weeds. Pesticides are routinely prescribed to manage those problems. Moreover, monocultural systems (in which only a single crop is grown in a field) result in reduced populations of natural predators and parasites, which can exacerbate existing pests and allow new ones to develop. Some environmentalists have described intensively managed agricultural systems as being a pesticide treadmill, because they rely on pesticides, often in increasing quantities, to deal with unanticipated pests that emerge as “surprises.”
The use of pesticides in Canadian agriculture has increased greatly in recent decades. Herbicide was applied to 26.7-million hectares of farmland in 2011, a three-fold increase over 1971 (Table 14.10). Insecticide and fungicide were applied to 8.7-million hectares, an 11-fold increase. Overall, pesticide use in North American agriculture increased by about ten-fold between 1945 and 1989 (Pimentel et al., 1992). Interestingly, during that same period, crop losses (to insects only) actually increased somewhat, from about 7% during 1941-1951 to 13% during 1951-1974 (Hayes, 1991). These trends, which might seem to contradict each other, may be due to such factors as the development of tolerance by some pests to pesticides, the emergence of new pests because of accidental introductions, changes in predator-prey relationships caused by pesticide use, and the introduction of new crop varieties that are vulnerable to pests.
Agricultural damage caused by arthropod pests varies greatly. Sometimes there is direct competition with humans for a food resource, as when insects defoliate crops in fields or attack stored food. Such depredations can sometimes obliterate agricultural yield, as can happen during a severe infestation of locusts. More commonly, however, insects reduce the yield only somewhat.
In some cases, however, even minor damage by pests can make the produce unsaleable. This can be the case of damage caused to apples by the codling moth (Carpocapsa pomonella). “Wormy” apples with larvae of this insect are not saleable to consumers, and up to 90% of the fruit in unsprayed orchards may be infested (McEwen and Stephenson, 1979). Even a minor discoloration of fruit, such as apple scab and russeting of oranges (neither affect the productivity or nutritional quality of the crop) are considered unacceptable by many consumers. Therefore, seemingly unimportant crop damage can be a critical economic consideration for farmers and the food industry. As with some potentially life-saving drugs, pesticides are over-prescribed for some uses.
Much pesticide use in agriculture is targeted against weeds, which interfere with crop plants by competing for limited resources of light, water, and nutrients. (Of course, “weediness” is partly a matter of context – in other situations, some weed species have positive attributes.) It is well known that weeds, if abundant, can cause large decreases in the productivity of agricultural crops, even by 50-90%. This is the reason why farmers have always taken measures to reduce the abundance of weeds, initially by hand-pulling or hoeing them and later by mechanical cultivation (ploughing) to disrupt their growth. More recently, chemical herbicides have been widely used to control agricultural weeds. In the United States, for example, herbicide is used on 85% of the planted area for most crops (Gianessi and Sankula, 2003). Crops receiving the most herbicide are canola (on 99% its cultivated area), dry beans (99%), carrot (98%), maize (98%), rice (98%), sugarbeet (98%), peanut (97%), green bean (96%), soybean (96%), tomato (96%), blueberry (95%), citrus (95%), cotton (95%), and potato (93%). Herbicide use in wheat is relatively low (55%) because mechanical cultivation during sowing is effective in reducing weeds for this crop.
According to Gianessi and Sankula (2003), if herbicide use were discontinued for the 40 crops they studied, then weed management would have to rely on increased mechanical cultivation and manual weeding. They calculated that would have an annual cost of US$14 billion, about double that of herbicide use ($6.6 billion per year). If herbicide use were discontinued and replaced by alternative practices, they estimated that 35 of the 40 crops studied would suffer a decline in productivity, by an average of 21% (range of 5-67%). The productivity loss would have a value of about $21 billion per year (including $13 billion in lost productivity and $8 billion in increased costs of management). Note, however, that the “direct costs” of herbicide use cited above ($6.6 billion/yr) do not include the value of environmental damage that might be caused, for instance, by toxicity to non-target plants, wildlife, or agricultural workers. It must be remembered that non-herbicidal methods of weed control also cause environmental damage, particularly mechanical cultivation, which increases soil erosion and compaction. No-till management systems, which greatly reduce the rate of erosion, must rely on the use of herbicide to control weeds.
Of course, the weeds must be vulnerable to the toxicity of the herbicide being used against them, while the crop plant must be tolerant. Some herbicides are toxic to broad-leaved weeds (dicotyledonous plants) but not to corn, wheat, barley, or other crops in the grass family (monocotyledonous plants). Consequently, herbicides are widely used in grain agriculture in North America. For instance, about 98% of the maize and rice acreage is treated. Some important diseases of agricultural plants can be managed with pesticide. Sometimes, insecticide is sprayed to control arthropod vectors of microbial diseases. More commonly, fungicide is used to control pathogenic fungi such as late blight (Phytophthora infestans) of potato, apple scab (Venturia inequalis), powdery mildew (Sphaerotheca pannosa) of peach, and seedrot and damping-off of many crop species (Pythium spp.). Fungicide also helps to prevent the spoilage of stored crops by fungi such as Aspergillus flavus, which can grow in stored legumes, grains, and nuts, producing deadly aflatoxins that make foods poisonous to humans and livestock.
Pesticides in Forestry
The use of pesticides in forestry raises a great deal of controversy, often more so than use of the same chemicals in agriculture. The controversy partly concerns damage that may be caused to the many native species that are exposed to forestry sprays, compared to mostly aliens in agriculture. In addition, spraying in forestry is mostly done by government agencies and large companies, while in agriculture it often involves individual farmers working a family farm. Most people have greater empathy for individuals than for big government or big business, and this can influence their opinions about pesticide use.
Pesticides are used in forestry mainly to control epidemics of defoliating insects and to manage weeds in re-forested areas and plantations. The largest insecticide spraying campaigns have been undertaken against spruce budworm (Choristoneura fumiferana) in New Brunswick, where a cumulative area of about 49-million hectares was sprayed between 1952 and 1992 (this is examined later as a case study). Other large spray programs have included those against gypsy moth (Lymantria dispar, an introduced pest that defoliates many tree species), hemlock looper (Lambdina fiscellaria), and bark beetles (especially species of Ambrosia and Ips).
Pesticides in the Home and Horticulture
Pesticides are commonly used in and around homes. For instance, insecticide may be used to kill bedbugs and cockroaches, and rodenticide to poison rats and mice. As well, large amounts of pesticide are used in horticulture. Herbicides are especially widely applied, mostly to achieve the grass-lawn aesthetic that many homeowners seek. To this end, herbicide is used to kill broad-leaved plants such as dandelion and plantain. The “weed” in common “weed-and-feed” lawn preparations is the herbicide 2,4-D, dicamba, or mecoprop.
Some of the most intensive pesticide usage occurs in the management of golf courses, particularly on putting greens where the lawn quality must be very consistent. Fungicide is used in especially large amounts to prevent turf-grass diseases. On a per-unit area basis, the use of pesticides on putting greens can be more intensive than almost any in agriculture.
Environmental Effects
Pesticide applications are intended to manage the impacts of pests by reducing their abundance and damage to below an economically or aesthetically acceptable threshold. This objective can sometimes be achieved selectively, thereby avoiding non-target damage. For example, rodenticide can be used judiciously to kill rats and mice around the home, while minimizing toxic exposures to non-target cats, dogs, and children (although risk is never eliminated).
More typically, however, pesticide use involves a less-selective broadcast application, usually by spraying. A crop-dusting aircraft or tractor-drawn sprayer is often used, which results in many non-target species being exposed to the spray. The non-target organisms may live on the sprayed site, or they may be off-site and suffer exposure from aerial or aquatic drift of a pesticide. Non-target exposures include both direct contact with a sprayed pesticide as well as indirect exposure through the food web.
The ecotoxicological risk that is inherent in an exposure to pesticide (and to other chemicals) is influenced by a complex of variables, as we previously examined in Chapter 15. Several points should be considered when interpreting exposures of non-target organisms (including people) to pesticides and other chemicals:
- All chemicals are potentially toxic
- Not all exposures to potentially toxic chemicals result in poisoning (because organisms are to some degree tolerant to pesticides and other chemicals)
- Some pesticides and some naturally occurring chemicals are extremely toxic to many organisms, including humans
- Humans are subject to both involuntary and voluntary exposures to certain toxic chemicals (the latter includes prescription and recreational drugs)
Of course, pesticides vary enormously in their toxicity. Herbicides, for example, are extremely toxic to at least some plants, but not necessarily to animals, which differ in important physiological respects from plants. In contrast, most insecticides and rodenticides are toxic to a wide range of animals and can cause non-target poisoning of diverse species, including humans.
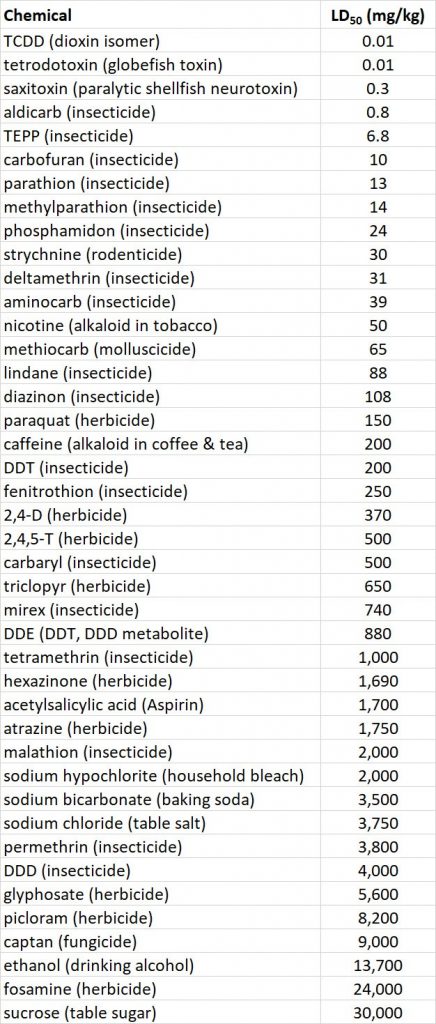
The acute toxicity of a chemical to animals is defined by its LD50, or the dose needed to kill one-half of a test population that is exposed through food, water, or air. The oral LD50 for rats is an indicator of acute toxicity to mammals. Rats are widely used in toxicological research and are similar to humans in many aspects of their physiology. Table 22.2 compares the acute toxicity of a wide range of pesticides and some other chemicals, using rat oral LD50 (see also Table 15.3). Note that some of the most poisonous chemicals listed are natural biochemicals, such as saxitoxin, a potent neurotoxin produced by certain marine algae. Others are chemicals to which many people expose themselves in their pursuit of pleasure, such as nicotine, the addicting alkaloid in tobacco.
Table 22.2. Acute Toxicity of Various Chemicals to Rats. The oral LD50, measured in milligrams of chemical per kilogram of body weight, is the amount required to kill 50% of a trial population of rats, exposed through their food in a controlled laboratory test. Source: Modified from Freedman (1995)
By poisoning organisms, pesticides may also cause habitat changes to occur, which can indirectly affect many species. For example, herbicide kills plants and thereby changes the habitat of animals, perhaps depriving herbivores of their preferred foods. Similarly, broad-spectrum insecticides kill large numbers of arthropods, which reduces the amount of food available for birds and other animals. These and other indirect effects of pesticide use can result in ecological damage, in addition to the directly toxic effects.
In the remainder of this chapter, we will examine several case studies of particular uses of pesticides. These are useful in illustrating the broader principles and patterns of the ecological damage caused by these chemicals.
DDT and Related Organochlorines
The first case study involves DDT and related organochlorine insecticides, such as DDD, dieldrin, and aldrin. These chemicals were once widely used in Canada and most other developed countries. Although these organochlorines were banned here in the early 1970s, they continue to be used in some less-developed nations.
DDT and its relatives are persistent in the environment. Consequently, even though these chemicals have not been used in Canada for several decades, there are still substantial residues in the ecosystems of our country. In part, this is also a result of the continued use of these organochlorines in some tropical countries, because small amounts of residue from those ongoing uses are transported to high-latitude countries by global cycling processes. In addition, organochlorines are more persistent in cooler environments than in warmer ones. As a result, these and some non-insecticidal organochlorines (such as PCBs and dioxins) are still important contaminants in Canada.
DDT was first synthesized in 1874, but its insecticidal properties were not discovered until 1939. Its first important use was during World War II in programs to control body lice, mosquitoes, and other disease vectors. DDT was quickly recognized as being an extremely effective insecticide, and it became widely used in agriculture, forestry, and against malaria. The use of DDT peaked in 1970, when 175-million kilograms were manufactured globally. Soon afterward, developed countries began to ban most uses of DDT because it was found to be causing ecological damage, including the contamination of people and their food web. Some researchers thought this contamination could be causing illnesses, such as increases in cancer and liver disease. However, DDT use has continued in some tropical countries, mostly against mosquito vectors of disease.
However, even in those countries the use of DDT and other organochlorine insecticides has been decreasing. This is partly because many pests have developed a genetically based tolerance of these chemicals (sometimes known as resistance), which decreases their effectiveness as pesticides. The development of tolerance is an evolutionary process in which exposure to a toxic substance selects for resistant individuals within a genetically variable population (see Chapters 6 and 15). Although tolerant individuals are normally rare in unsprayed populations, they may become rapidly dominant in sprayed habitat. If the insecticide does not kill them, they survive to reproduce, and pass on the genes for tolerance to their offspring. More than 500 species of insects and mites have populations tolerant to at least one insecticide, and there are more than 100 fungicide-resistant plant pathogens, 55 herbicide-tolerant weeds, and five rodents resistant to anticoagulants (NRC, 1986; Winston, 199; Landis et al., 2002).
Several physical and chemical properties of organochlorines have an important influence on their ability to cause ecological damage. First, they have an extended persistence, or a tendency to remain chemically unchanged in the environment because they are not easily degraded by microorganisms or by physical agents such as sunlight or heat. For example, DDT has a half-life in soil of 3-10 years. The primary breakdown product of DDT is the closely related organochlorine DDE, which has a similar persistence.
In addition, DDT and related organochlorines are essentially insoluble in water and so cannot be “diluted” into that abundant solvent. Instead, these chemicals are highly soluble in fats (or lipids), which occur mostly in organisms. Therefore, DDT and related organochlorines have a strong affinity for organisms, and they accumulate in living things in strong preference to the non-living environment – this process is called bioconcentration.
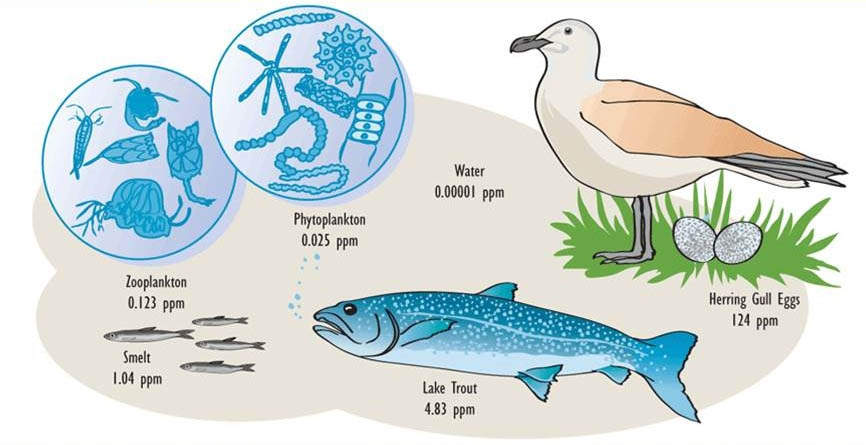
Moreover, organisms are highly efficient at assimilating organochlorines that are present in their food. As a result, predators at the top of the food web develop the highest residues of organochlorines, particularly in their fatty tissues (this is known as biomagnification or food-web magnification). Both bioconcentration and food-web magnification are progressive with age, so the oldest individuals in a population are the most contaminated (see In Detail 18.1).
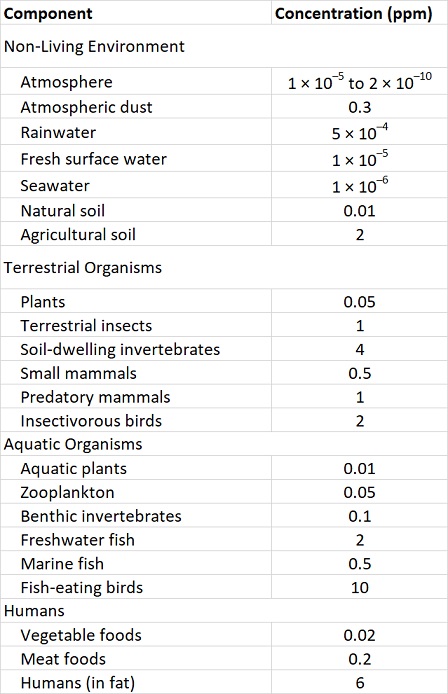
These properties of organochlorines are illustrated in Figure 22.1 and Table 22.3. Note that the concentrations are miniscule in air, water, and non-agricultural soil, compared with the much higher residues that occur in organisms. Note also that concentrations in plants are lower than in herbivores, and that residues are highest at the top of the food web, such as in predatory birds and humans.
Table 22.3. Typical Residues of DDT in the 1960s and 1970s. Sources: Data from Edwards (1975) and Freedman (1995).
Another characteristic of organochlorines is their ubiquity – their residues occur in all organisms throughout the biosphere. This widespread contamination occurs because organochlorines enter a global cycle and become widely dispersed in the bodies of migrating organisms and in the atmosphere by evaporation and in wind-eroded dust. Residues of DDT are found even in organisms in Antarctica, far remote from areas where it was ever used. In one study in that far-southern region, the concentration of “total DDT” (almost all of which occur as the metabolic residue, DDE) in the fat of skuas (a marine bird, Catharacta maccormicki) was 5 ppm (or 5 µg/g). Smaller residues (< 0.44 ppm) occurred in birds feeding lower in the food web, such as fulmar (Fulmarus glacialoides) and macaroni penguin (Eudyptes chrysolophus) (Norheim et al., 1982).
Although organochlorine residues are ubiquitous in the biosphere, much higher concentrations occur in animals that live close to areas where these chemicals have been used, such as North America. Because marine mammals feed at or near the top of their food web and are long-lived, they can have extremely high residues of organochlorines. For example, harbour porpoises (Phocoena phocoena) in Atlantic Canada have had DDT residues as high as 520 ppm in their fat (Edwards, 1975). High residues of organochlorines also occur in top-predator birds, especially raptors (such as eagles, falcons, hawks, and owls). Prior to the banning of DDT, residues averaged 12 ppm (with a maximum of 356 ppm) in a sample of 69 bald eagles (Haliaeetus leucocephalus), up to 460 ppm among 11 western grebes (Aechmophorus occidentalis), and up to 131 ppm among 13 herring gulls (Larus argentatus) (Edwards, 1975).
Intense exposures to organochlorines caused important ecological damage, including bird poisonings. During the 1950s and 1960s, bird kills resulted when DDT was sprayed in urban areas to kill the beetle vectors of Dutch elm disease, caused by a fungal pathogen (Ceratocyctis ulmi) that was accidentally introduced to North America from Europe. The fungus is transported between trees by bark beetles, which can be controlled to some degree by the use of insecticide. Spraying for this purpose was intensive, and typically involved an application of 0.7-1.4 kg of DDT per tree. Birds feeding on invertebrates in treated areas were exposed to lethal doses. One study in New Hampshire found 117 dead birds in a 6 ha spray area, and estimated that 70% of the breeding robins (Turdus migratorius) had been killed (Wurster et al., 1965). So much avian mortality occurred in sprayed neighbourhoods that bird song was markedly reduced – hence the title of Rachel Carson’s 1962 book, Silent Spring (In Detail 22.3).
In addition to acute poisoning caused by organochlorines in sprayed areas, more insidious damage was occurring over large regions. Many species experienced long-term chronic toxicity, even well away from sprayed areas. It took years of population monitoring and ecotoxicological research before organochlorines were identified as the cause of this widespread damage. In fact, we can view the chronic poisoning of birds and other wildlife as an ecological “surprise” that occurred because scientists (and society) had no experience with the long-term effects of persistent, biomagnifying organochlorines.
Raptorial birds were among the prominent victims of organochlorine insecticides. These birds are vulnerable because they are top predators and accumulate high residues of organochlorines. Breeding populations of various raptors suffered large declines. Severely affected species included the peregrine falcon (Falco peregrinus), osprey (Pandion haliaetus), bald eagle (Haliaeetus leucocephalus), and golden eagle (Aquila chrysaetos) (see Canadian Focus 22.1). In all cases, these species were exposed to a “cocktail” of organochlorines, including the insecticides DDT, DDD (both are metabolized to DDE), aldrin, dieldrin, and heptachlor, as well as PCBs, a group of non-insecticidal compounds with many industrial uses. Researchers have investigated the relative importance of these various organochlorines in causing the population declines of raptors. It appears that DDT may have been the more important toxin to birds in North America, while cyclodienes (particularly dieldrin) were more influential in Britain (Cooper, 1991; Moriarty, 1999).
Damage to raptors was mainly associated with chronic effects on their reproduction, rather than toxicity caused to adults. Reproductive damage included the production of thin eggshells that would break under the weight of an incubating parent, high mortality of embryos and nestlings, and abnormal adult behaviour. The numbers of fledged chicks declined, and that resulted in rapid population declines.
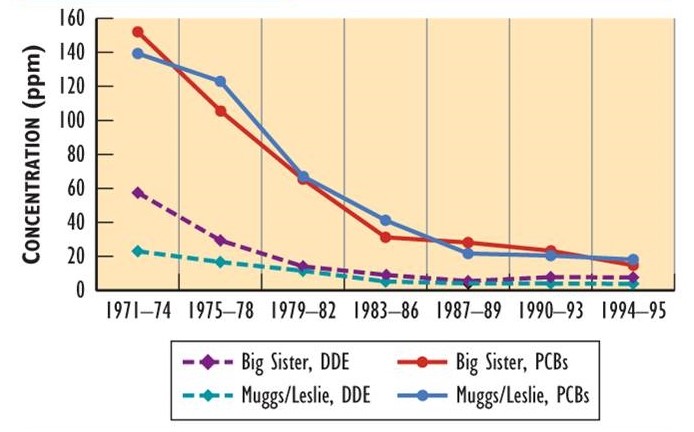
Since the bans of DDT and other organochlorines in North America, their residues in wildlife have been progressively decreasing. Data showing this decline have been obtained by analyzing eggs of herring gulls (Larus argentatus) breeding on the Great Lakes (Figure 22.2). Although eggs from various places differ in their residues (partly depending on local sources), they all exhibit large decreases in DDE and PCB. Decreases in residues have also occurred in double-crested cormorants (Phalacrocorax auritus).
Figure 22.2. Changes in Organochlorine Residues in Bird Eggs. Eggs of herring gull have shown decreasing residues since the use of DDT, PCBs, and other persistent organochlorines was banned in North America in the early 1970s. Muggs/Leslie are breeding sites in the Toronto waterfront, while Big Sister Island is in Green Bay in Lake Michigan. Residues are measured in ppm. Sources: Data from Bishop and Weseloh (1990), Environment Canada (1993), and Ryckman et al. (2005).
In Detail 22.3. Silent Spring Rachel Carson, an American biologist, wrote many scientific articles and several books, the most famous of which, Silent Spring, was published in 1962. Silent Spring was aimed at a popular audience, and it was a lively and controversial indictment of pesticide use as it was practised at the time, particularly the use of DDT and other organochlorine insecticides. Silent Spring was written to warn society about the known and potential dangers that these pesticides pose to wildlife, and also to people through contamination of their food. Silent Spring achieved that objective and, in fact, was a literary bombshell that caused an eruption of public awareness about pesticide issues.
Although DDT and its organochlorine relatives were clearly useful in killing pests, Carson described how they were also causing extensive mortality to non-pest arthropods, and also to birds, mammals, and other wildlife. She also warned that people were being widely exposed to organochlorines, with significant residues being found, for example, in the milk of nursing mothers. She noted, “For the first time in the history of the world, every human being is now subjected to dangerous chemicals, from the moment of conception until death.” Although little was known about the subject at the time, Carson warned that the chronic, low-level exposure of people to organochlorines was potentially dangerous.
A best-seller, Silent Spring stirred up an enormous controversy about the effects of anthropogenic chemicals in the environment. Companies that manufactured pesticides mounted their own information and advertising programs. They attempted to discredit Carson by labelling her as an irresponsible agitator and by claiming that she did not represent the views of most scientists. In fact, some of the technical details of Carson’s analysis were later found to be incorrect, but this is not surprising considering the incomplete understanding at the time about pesticides and their environmental impacts. Nevertheless, the essential thesis of Silent Spring was that organochlorine insecticides were widely contaminating organisms and the environment, were persistent, and were causing extensive damage. In large part, these assertions were correct.
Unfortunately, Rachel Carson died an early death from cancer in 1964, just as the message of Silent Spring was becoming widely recognized. Today, Carson is known as one of the most influential environmentalists in history, a pioneer who deserves much of the credit for the birth of the environmental movement during the mid-1960s. Like all environmentalists, Rachel Carson promoted an ethic of human responsibility for taking care of the biosphere and its species.
The Case of Carbofuran
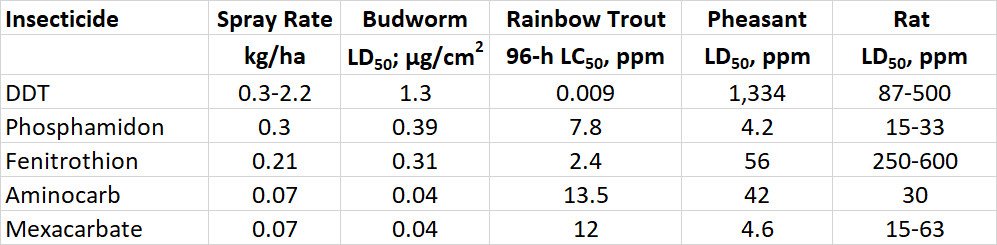
Important replacements for DDT and related organochlorines have been organophosphate and carbamate insecticides. These poison insects and other arthropods by inhibiting a specific enzyme, acetylcholinesterase (AChE), which is critical in the transmission of nerve impulses. Vertebrates such as amphibians, fish, birds, and mammals are also sensitive to poisoning of their AChE system. In all of these animals, acute poisoning by organophosphate and carbamate insecticides causes tremors, convulsions, and ultimately death.
Carbofuran is a carbamate insecticide that can be used for many purposes in agriculture. One formulation is a liquid suspension that can be diluted in water and then broadcast-sprayed against pests such as grasshoppers and leaf beetles. It is also available in a granular formulation, in which the insecticide coats particles of grit and is sown along with seeds to protect tender seedlings from insect damage. The granular formulation has been commonly used when sowing canola and maize.
Unfortunately, wildlife is exposed to toxic doses of carbofuran when either of these formulations is used. For example, if not all of the carbofuran granules are buried in the planting furrows, they remain exposed on the surface (Mineau, 1993). In one method of seeding, used for corn in Ontario, 15-31% of the granules remained exposed on the surface, or 515-1065 exposed granules per metre of furrow. Methods used to plant canola in western Canada often left about 5% of the granules on the surface. The exposed granules may be ingested by seed-eating birds, which require hard particles of that size as “grit” for macerating hard-coated seeds in their muscular gizzard. The carbofuran is extremely toxic: consumption of only 1-5 granules can kill a small bird. Raptors and mammals are secondarily poisoned if they scavenge the dead bodies.
In addition, fields treated with carbofuran may become flooded during the spring and autumn, and the surface water may then contain large residues of the insecticide. This is particularly the case if the soil and water are acidic, which greatly reduces the rate of breakdown of carbofuran into less toxic chemicals.
Of all the pesticides used recently in agriculture, carbofuran has probably caused the most non-target mortality of birds and other wild animals. Even though there is no systematic program for reporting bird kills caused by pesticide use in Canada or the United States, a large number of toxic incidents were documented for carbofuran (Mineau, 1993), a few of which were:
- More than 2,000 Lapland longspurs (Calcarius lapponicus), a seed-eating finch, were killed after eating carbofuran granules in a freshly planted canola field in Saskatchewan in May 1984
- About 1,200 birds, mostly savannah sparrows (Passerculus sandwichensis), were killed in by granular carbofuran in turnip and radish fields in British Columbia in September 1986
- More than 1,000 green-winged teal (Anas carolinensis) were killed within hours of landing in a flooded turnip field in British Columbia in the autumn of 1975
- At least 50 mallards (Anas platyrhynchos) and pintails (A. acuta) were poisoned in a flooded field in British Columbia in December 1973
- 2,450 dead widgeon (Mareca americana) found one day after spraying of an alfalfa field in California in March 1974
These examples are only a small fraction of the known bird kills caused by the routine use of carbofuran in agriculture. There are also, of course, larger numbers of unreported incidents. Because carbofuran use in agriculture carries such a well-known risk of poisoning birds and other wildlife, ecologists and environmentalists lobbied vigorously to have its registration withdrawn for those uses, or at least more tightly controlled. In 1993, the U.S. Environmental Protection Agency banned the sand-based granular formulation of carbofuran, except for some relatively minor uses and a major one (with rice crops) for which there was no suitable alternative. In 1996, Agriculture Canada, the federal agency that regulates pesticide use, prohibited most uses of carbofuran in liquid suspension, as well as all granular formulations. These were positive actions in terms of pesticide regulation, although in the view of many wildlife toxicologists it took an excessively long time for those necessary steps to be taken.
Canadian Focus 22.1. Organochlorines and the Peregrine Falcon The most famous avian victim of organochlorines was the peregrine falcon, whose population declines were first noticed in the early 1950s in North America and Western Europe (Peakall, 1990; Freedman, 1995). By 1970, peregrines in eastern North America (the subspecies Falco peregrinus anatum) had stopped reproducing and were critically endangered. At the same time, the tundrius subspecies of the Arctic was rapidly declining. Only the pealei subspecies that breeds on islands off British Columbia and Alaska had normal reproduction success and a stable population.
The pealei falcons do not migrate. Moreover, they live in a region where pesticides are not used and mainly feed on non-migratory seabirds. In contrast, anatum peregrines breed in a region where organochlorines were widely used, and they fed on contaminated prey. The tundrius falcons breed in a northern wilderness where pesticides are not used, but they winter in Central and South America where their food was contaminated by organochlorines, as was their prey of migratory waterfowl and shorebirds in the Arctic. Studies by wildlife toxicologists found that populations of peregrine falcons with high organochlorine residues were laying thin-shelled eggs and suffering other kinds of reproductive impairment. This damage was causing their populations to collapse. By 1975, the anatum birds had become extirpated in eastern North America, while the arctic tundrius birds had declined to only about 450 pairs from historical levels of more than 5-thousand pairs.
Fortunately, many countries (including Canada, the United States, and most other developed nations) banned further use of DDT and most other organochlorines in the 1970s. This resulted in a marked decrease in residues in the food of peregrines and other raptors, which allowed their populations to stabilize or recover. By 1985, arctic peregrines were increasing in abundance, and small breeding populations had re-established in more southern regions.
The recovery was greatly enhanced by a program (funded in Canada by the Canadian Wildlife Service) that bred peregrines in captivity to provide young birds for release into the former range of the anatum subspecies. Several thousand young peregrines were released in Canada and the U.S., and many of the birds survived and bred. Some of them were released in cities, where tall buildings provide cliff-like nesting habitat and there are abundant pigeons and other urban birds as prey. Thanks to declining residues of organochlorines resulting from bans on the use of these chemicals, the peregrine falcon is on its way back.
Image 22.1. The peregrine falcon is a species that suffered widespread population declines as a result of the ecotoxicological effects of DDT and other organochlorines. Source: Dennis Jarvis, Wikimedia Commons; http://commons.wikimedia.org/wiki/File:Falco_peregrinus_-Nova_Scotia,_Canada_-eating-8.jpg
Diazinon and Monocrotophos
Other modern insecticides are also poisoning Canadian birds. Diazinon, an organophosphate insecticide, has caused numerous cases of mass mortality. For instance, in the late 1980s, there were at least five events in which entire flocks of Canada goose (Branta canadensis) were killed when they fed on grass on golf courses in southern Ontario that had been treated to reduce infestations of turf insects (Mineau, 1999). The geese died within minutes, as is typical of acute poisoning by AChE inhibitors. Similar toxic events have occurred in the United States, including one in which 700 Brant geese (Branta bernicla) were killed on a golf course in New York. Diazinon has now been banned for use on golf courses in the U.S., but it can still be used in Canada for that purpose and also for horticultural and agricultural purposes, although its use is declining.
In 1996, it was discovered that agricultural use of monocrotophos and other organophosphate insecticides against grasshoppers in Argentina was killing large numbers of Swainson’s hawks (Buteo swainsoni). This raptor breeds in western Canada and the U.S. and migrates to winter on the pampas of South America. Populations of this hawk had been declining for several years. However, it was not until some birds were fitted with satellite transmitters and followed to Argentina that wildlife toxicologists discovered a probable cause of the decline – poorly regulated use of monocrotophos on the wintering grounds. Field studies in Argentina discovered that more than 20-thousand Swainson’s hawks had been killed in just one agricultural area (other regions were not surveyed), out of a total breeding population of only 400-thousand (of which up to one-quarter breed in Canada).
Monocrotophos is extremely toxic to birds, although it is not persistent in the environment. Because of the risk of ecological damage, monocrotophos has been banned in the United States and was never registered for use in Canada. In Argentina, however, the insecticide could be legally used. Swainson’s hawks were exposed to lethal doses of monocrotophos when they fluttered behind spray tractors to feed on grasshoppers flushed by the machinery, and also when they later fed on insecticide-contaminated prey. Argentina has since banned monocrotophos, replacing its applications with pyrethroids.
Neonicotinoids
Perhaps the most contentious pesticide-related issue of recent years (this was written in 2015) is the use of neonicotinoid insecticides agriculture (Jeschke et al., 2011; Mineau and Palmer, 2013; van der Sluijs et al, 2014). These are a class of synthetic AChE-inhibiting insecticides that are chemically similar to nicotine, the key alkaloid in tobacco. Neonicotinoids are much less toxic to vertebrate animals that carbamate and organophosphate insecticides, but they are persistent and broad-spectrum poisons to non-target arthropods. The “neonic” group includes a variety of compounds, with imidacloprid recently being one of the most widely used insecticides in the world.
Neonicotinoids are a systemic insecticide. The chemical can be applied with irrigation water, as a water-based spray, or as a coating on seed intended for planting. In all of those cases, the neonic is absorbed and then distributed throughout the plant to confer protection against herbivorous insects. These have become popular uses – since the introduction of neonics in the early 1990s, they have grown to account for several billions of dollars of annual sales. Neonicotinoids are used on a wide range of crops. In the United States, they are used on 95% of the canola and maize acreage, and at least half of cotton, sorghum, soybean, and sugar beet. They are used on many fruit crops, including almonds, apples, berries, cherries, grapes, oranges, and peaches, as well as vegetables such as leafy greens, potatoes, and tomatoes, and even cereal grains. They are also used as a wood preservative.
Recently, the use of neonicotinoids has been linked to some important environmental problems. One is the general decline in pollinating insects, which are vital both to agricultural production and to natural ecosystems. The agricultural linkage involves that fact that most commercial fruits, ranging from apples to zucchinis, rely on insects to pollinate their flowers so that fruit development can occur. Honeybees are especially important in this regard, yet these vitally important pollinators have been badly declining because of a syndrome referred to as colony collapse disorder. The cause of that damage is not exactly known, but neonics are suspected as having a contributing influence. Another indirect effect of the widespread use of neonics could be declines of some bird species due to a reduction of their arthropod food base (Hallmann et al., 2014).
As a result of the growing concerns about the environmental effects of neonicotinoid insecticides, many countries have been restricting or banning their use. In 2013, a study by the European Food Safety Authority reported an unacceptably high risk to honeybees from many uses of neonics, and in 2014 a critical integrated study was published (van der Sluijs et al, 2014). In 2014, in response to this and other research, 15 of the 27 European Union member states voted to restrict the use of three neonicotinoids (clothianidin, imidacloprid, and thiamethoxam) for two years while additional studies were undertaken. The U.S. Environmental Protection Agency is reviewing their registration, and Canadian authorities are monitoring those international developments.
Pest Problems in Forestry
Pesticides are used much more extensively in agriculture than in forestry (about 80% of pesticide sales in Canada are for use in agriculture, 12% for domestic and industrial use, and 2% for forestry purposes; Environment Canada, 1996). However, forestry case studies better illustrate many of the ecological effects of pesticide use because the treated habitats support mainly native species and natural or semi-natural ecosystems. In contrast, agricultural habitats are dominated by non-native species and are intensively managed, making them less amenable to the examination of some of the ecological effects of pesticides.
Image 22.2. This is an area of intensive forest damage caused by spruce budworm on the highlands of Cape Breton Island. The living trees fringing the bog are black spruce, which is resistant to the budworm. The extensive area of dead trees was dominated by balsam fir, a vulnerable species. This photograph was taken several years after the collapse of the outbreak. Source: B. Freedman.
Spruce Budworm
The largest insecticide spray programs in forestry have been mounted against spruce budworm (Choristoneura fumiferana), particularly in New Brunswick. Because the spraying occurs over extensive natural forests, whose biodiversity consists of native species, it represents an excellent case study to examine the ecological effects of insecticide use. Spruce budworm is a native moth whose larvae are pests of fir- and spruce-dominated forest. An infestation can affect tens of millions of hectares, and trees are killed after several years of defoliation. Mature stands dominated by balsam fir (Abies balsamea) are particularly vulnerable. White spruce (Picea glauca) is also a preferred food, but red spruce (P. rubens) and black spruce (P. mariana) are less apt to suffer lethal damage. Spruce budworm is always present in small populations in its fir-spruce habitat, but it occasionally irrupts to an enormous abundance and becomes a pest. Under normal conditions, only about five larvae may occur on each conifer tree, but this increases to 2-thousand at the beginning of an irruption, and to more than 20-thousand during the peak. A local outbreak is typically sustained for 6-10 years, and then collapses. Studies in Quebec have shown that outbreaks have occurred at an average interval of about 35 years (Blais, 1985). An outbreak is typically synchronous (occurring at the same time) over an extensive area of vulnerable forest, although there are large variations in the abundance of budworm among stands. The exact reasons for the irruptions are not known, but they may involve several years of warm, dry weather in the springtime, which favours the survival of larvae.
Forest Damage
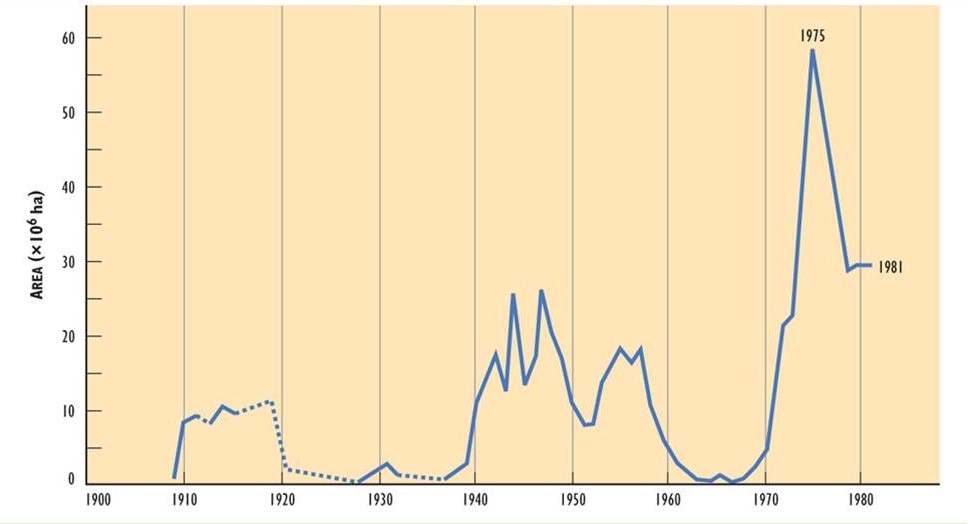
It appears that the extent of damage caused by spruce budworm may have increased during the three outbreaks of the twentieth century. The outbreak that began in 1910 affected about 10-million hectares, one starting in 1940 involved 25-million ha, and another in 1970 affected more than 55-million ha (Figure 22.3). The enlarging areas of infestation may be related to an increase in the extent of vulnerable fir-spruce forest, possibly due to the following influences:
- regeneration of conifer stands on abandoned farmland, particularly since the 1920s
- protection of forests from wildfire
- forestry practices such as clear-cutting
- spraying of infested stands with insecticide, which may help maintain the habitat in a condition suitable for budworm
Figure 22.3. Forest Area Defoliated by Spruce Budworm in the Twentieth Century. The area corresponds to stands suffering severe or moderate defoliation. Source: Modified from Kettela (1983).
Enormous damage has been caused by budworm to economically important forest resources. During the most recent outbreak (1971-1984), tree mortality was equivalent to more than 38-million cubic metres of saleable timber. During the peak of the infestation, substantial tree mortality occurred over about 26.5-million hectares in eastern Canada (Ostaff, 1985). The rapid development of an infestation can be illustrated by the case of Cape Breton Island (Ostaff and MacLean, 1989). No defoliation by budworm was observed in 1973, but in 1974 there was moderate-to-severe defoliation over 165-thousand hectares. This increased to 486-thousand ha in 1975, and to 1.22-million hectares in 1976, when essentially all of the vulnerable fir-spruce forest was infested.
An irruption of budworm can last for 10 or more years, with the damage increasing over time. During the first two years of severe defoliation on Cape Breton, 4% of the balsam fir trees died. The cumulative mortality increased to 9% after four years of heavy defoliation, 37% after six years, 48% after eight years, 75% after 10 years, and 95% after 12 years (Ostaff and MacLean, 1989). Across eastern Canada, tree mortality averaged 85% in mature fir-dominated stands, 42% in immature fir stands, and 36% in mature spruce stands (MacLean, 1990).
Mature trees are much more vulnerable to budworm than are smaller immature ones, which commonly survive an outbreak. Consequently, the understorey of a damaged stand typically contains a dense population of small fir and spruce. Known as advanced regeneration, this is important in re-establishing the next fir-spruce forest after an infestation collapses. On Cape Breton, severely damaged stands typically had an advanced regeneration of 45-thousand small fir plus 3-thousand spruce per hectare, most of which survived the infestation (MacLean, 1988).
After the mature trees die and the canopy opens up, the small conifers grow rapidly and establish another fir-spruce forest, which becomes vulnerable decades later to another irruption of budworm. These observations suggest that, over the long term, the interaction between the forest and budworm can be viewed as an ecologically stable, cyclic succession. The natural cycle of disturbance and recovery has probably been occurring for thousands of years, although human influences may have increased its scale since the nineteenth century.
Of course, spruce budworm causes great economic instability in the forest-products industry, which is in competition with this moth for the fir-spruce resource. The periodic irruptions of budworm severely damage the forest, making it difficult for humans to plan their own orderly harvesting and management of the trees. Spraying is one way of dealing with this problem, because it can limit the defoliation and prevent some tree mortality. The objective of spraying is not to eradicate the budworm, but to decrease the damage it causes, and thereby maintain the forest resource and its dependent economy.
Image 22.3. This is a ground-level view of a stand damaged by spruce budworm on Cape Breton Island. Although the mature balsam fir trees have been killed, dense regeneration is occurring in the understorey. After 40-50 years, another mature forest will have developed, ready to be harvested by budworm or by humans. Source: B. Freedman.
Insecticide Spraying
After the Second World War, an early use of DDT was against spruce budworm. In 1953 alone, 804-thousand hectares of forest were sprayed in Quebec and New Brunswick. By 1968, when further use of DDT for this purpose was banned, a total of 15-million ha had been sprayed at least once (Ennis and Caldwell, 1991). In New Brunswick alone, 5.75-million kilograms of DDT were sprayed onto budworm-infested forest (Armstrong, 1985).
After the further use of DDT was banned in 1968, the insecticides used were fenitrothion and phosphamidon, both organophosphates, and aminocarb, a carbamate. Of these, fenitrothion was used most widely. By 1985, phosphamidon had been sprayed on 8.1-million hectares, aminocarb on 19-million ha, and fenitrothion on 64-million ha (these are sums of annual sprayed areas, with most treated stands receiving two sprays per year; Ennis and Caldwell, 1991). New Brunswick had the biggest spray program to “protect” the forest resource against budworm – up to 1985, a cumulative total of 69-million ha was treated, compared to 37-million ha in Quebec, 10-million ha in Maine, and 1.7-million ha in Newfoundland. The most extensive spraying in New Brunswick was in 1976, when 4.2-million hectares were treated. Afterward, spraying decreased to less than 1-million ha after 1985, to less than 0.5-million ha after 1990, and to zero after 1993 due to collapse of the budworm outbreak.
During the late 1980s, a biological insecticide based on the bacterium Bacillus thuringiensis var. kurstaki (abbreviated B.t.) began to displace the synthetic pesticides in budworm spraying. B.t. is toxic to most lepidopterans (moths and butterflies) and to some other insects, including blackflies and mosquitoes. Otherwise, this insecticide causes little non-target damage. Initially, budworm control using B.t. was variable in effectiveness, and the cost was high compared with fenitrothion. Since then, however, the effectiveness and cost of spraying B.t. have improved. This, along with concern about ecological damage caused by fenitrothion, has resulted in B.t. becoming the insecticide of choice in spray programs against budworm.
Non-Target Damage
The synthetic insecticides used against budworm caused a great deal of non-target mortality. Typical spray rates and toxicities for the insecticides are summarized in Table 22.4. DDT is the least toxic to budworm, but the most toxic to salmonid fish. Perhaps the most important reason for the 1968 ban on using DDT against budworm was the mortality caused to sportfish, particularly Atlantic salmon (Salmo salar) and brook trout (Salvelinus fontinalis).
Table 22.4. Acute Toxicity of Insecticides Used against Spruce Budworm. Typical application rates for forestry purposes are in kilograms per hectare. Acute toxicity to budworm larvae and selected vertebrate species was determined under laboratory conditions. The units of toxicity to budworm are in micrograms per square centimetre of body surface; for trout, data are ppm in water; and for pheasant and rat, data are ppm in food. Note: the 96-hr LC50 is the concentration in water that killed 50% of a population of trout after a 96-hour exposure. Source: Freedman (1995).
The insecticides that initially replaced DDT are more toxic to budworm and so could be sprayed at lower rates while achieving a similar degree of pest control. Although these insecticides are less toxic to fish than DDT was, they can be very poisonous to other animals. Fenitrothion and aminocarb, for example, are highly toxic to all arthropods, so their use results in an enormous kill of non-target insects and spiders, including many predators of budworm. One study estimated that a typical fenitrothion spray killed as many as 7.5-million individuals of hundreds of species of arthropods per hectare, although more than 90% of the dead biomass was budworm (Varty, 1975). Overall, a typical spray with fenitrothion caused a short-term decrease in arthropod biomass of 35%, and a decrease in total individuals of 50% (Agriculture Canada, 1993). However, studies found that the non-target damage to arthropods was temporary and long-term decreases in abundance were not detectable (Varty, 1975; Millikin, 1990). The post-spray recovery was due to recolonization from non-sprayed forest, along with increases by arthropods that survived the spraying.
Bird populations are unusually abundant in infested forest because budworm is a plentiful and nutritious food for insect-eating animals. In fact, some birds are uncommon except in budworm-infested forest. In one study, the breeding population of bay-breasted warbler increased from only 0.25 pairs/ha in non-infested forest to 30 pairs/ha during a budworm outbreak, while Tennessee warbler increased from 0 to 12.5 pairs/ha (Morris et al., 1958).
During an outbreak of budworm, most birds rely heavily on the larvae as food for raising nestlings. One study estimated that birds eat 89-thousand budworm larvae and pupae per hectare of infested forest, compared with 6-thousand/ha in stands without an irruption (Crawford et al., 1983). However, despite the enthusiastic effort, bird predation has no substantial effect on the abundance of budworm during an outbreak – birds consume only about 2% of the larvae. In essence, insectivorous birds are satiated by the extremely abundant food resource and are incapable of controlling the huge population of larvae. Predation by birds may, however, be important in reducing less-abundant budworm populations, and may help to lengthen the interval between outbreaks.
Because birds are abundant in a budworm-infested forest, they are exposed to any insecticide that might be sprayed. Even though some of the insecticides used against budworm are extremely toxic to birds, it has proved difficult to document actual damage to avian populations by spraying. First, it is extremely difficult to find dead or dying birds in forest habitat because they occur in a small density and are quickly scavenged by other animals. Even with all the insecticide spraying in New Brunswick between 1965 and 1987, the Canadian Wildlife Service has records of only 125 dead birds (Busby et al., 1989), which is a gross underestimate of the actual mortality.
In addition, it is difficult to detect population changes resulting from mortality of birds in forest. A census of forest birds is taken by mapping the locations from which male birds sing; this information is then used to determine the boundaries of their territories. The song censuses are conducted in the spring over a period of four to six weeks. During that time, bird populations are dynamic because migratory species are returning from their wintering grounds, and they arrive at different times. Moreover, if a territory-holding bird is killed by an insecticide spray, it may be quickly replaced from a “surplus” of non-breeding individuals that wander extensively, searching for suitable habitat that is not occupied by another of their species. Because of the temporal variations in bird abundance and the rapid replacement of killed individuals, it is difficult to document population changes caused by insecticide spraying.
Phosphamidon is very poisonous to birds (Table 22.4), and its use likely caused severe mortality of some species. One study suggested that as many as 376-thousand ruby-crowned kinglets were killed in New Brunswick during the 1975 spray season, mostly by phosphamidon (Pearce and Peakall, 1977). Because they forage high in the canopy, kinglets are particularly vulnerable to insecticide exposure during an aerial spray. The presumed damage to birds was the key reason why phosphamidon was banned for use against budworm after 1975.
Fenitrothion is less poisonous to birds, but it nevertheless has a small margin of toxicological safety during operational sprays. While a normal application appears to cause little avian mortality, exposure to a double spray, as commonly occurs with overlapping spray swaths, can be lethal. Studies of white-throated sparrows found much greater mortality and behavioural impairment after a double application of fenitrothion, compared with the normal spray rate (Busby et al., 1989).
Table 22.5 shows the effects of a fenitrothion spray on birds. When interpreting these census data, the trends in the sprayed habitat should be compared with those of non-sprayed forest. The comparison is necessary because the “pre-spray” census was conducted in mid-May, when many migratory birds had not yet returned to their breeding habitat. Consequently, the total avian abundance in the pre-spray census was much smaller than occurs later on. If the data are considered in this relative sense, they suggest that the fenitrothion spraying had no obvious effects on the abundance or species composition of the avian community. It is likely, however, that some birds were poisoned by fenitrothion during the spray and that the damage was not reflected in the census for the reasons we noted earlier.
Table 22.5. Effects of Fenitrothion on Bird Populations. The data show the effects of forest spraying with fenitrothion on breeding birds in the Gaspé region of Quebec. The operational spraying procedure is to treat stands twice, about one week apart. In this study, birds were censused for five days before spraying, then for seven days after the initial spray on May 21, 1976, and again for five days after the second spray on May 30. The sprayed stand (“Spr”) was treated with fenitrothion at 0.56 kg/ha, while the unsprayed reference stand (“Ref”) monitors changes that are unrelated to spraying. Bird density is expressed as numbers per 10 hectares. Only prominent species are listed. Source: Data from Kingsbury and McLeod (1981).
It should also be recognized that a budworm outbreak causes severe damage to the forest, and this affects the habitat of wildlife. Table 22.6 illustrates the effects of this habitat change on birds. Defoliation by budworm caused relatively little damage to Stand A, so the decreased population of some birds (such as solitary vireo and many warblers) was caused by a decrease in the availability of food because the outbreak had collapsed and budworm is a critical resource. In contrast, Stand B was intensely damaged by budworm, with large habitat changes that included many dead trees and a lush growth of understorey plants. In this stand, the decreased abundance of some birds (such as solitary vireo, Tennessee warbler, black-throated green warbler, blackburnian warbler, and bay-breasted warbler) resulted from changes in vegetation as well as a reduced availability of larvae as food. Note, in addition, that some birds (such as least flycatcher, magnolia warbler, and white-throated sparrow) do well in recently disturbed stands: they benefit from the habitat associated with budworm damage.
Table 22.6. Abundance of Birds During and After a Spruce Budworm Outbreak. The data are from a study in New Brunswick. Stand A was monitored for eight years during an infestation (1952-1959), and then for six post-outbreak years (to 1965). However, Stand A suffered little damage to its trees – the average age of balsam fir trees was 120 years in both sampling periods. Stand B was censused for five infestation years (1955-1959) and for five post-outbreak years (to 1964). Stand B suffered intense damage to its trees – the fir averaged older than 80 years when the infestation began, but < 10 years old afterward because so many mature trees had died. The bird data are in numbers per 40 hectares. Only prominent species are listed. Source: Data from Gage and Miller (1978).
Overall, it appears that the demonstrated ecological effects of post-DDT spray programs against spruce budworm were relatively short in duration and moderate in intensity (with the exception of the effects of phosphamidon on birds). Residues of such chemicals as fenitrothion and aminocarb are not long lived, and no magnification occurs in the food web. Although substantial mortality was caused to many non-target species, long-term decreases in their populations have not been documented (bearing in mind that such effects are hard to demonstrate, particularly at larger spatial scales). For example, although many individual birds have undoubtedly been poisoned by insecticide toxicity, measurable damage to their populations has not been demonstrated.
Spray Policies
Spraying insecticide on forest infested by spruce budworm has economic benefits that are associated with the protection of conifer trees, an important natural resource. As a result, decision makers and regulators in some provinces have considered spray programs to be necessary. Many environmentalists, however, come to different conclusions about the benefits and costs of spraying, because they valuate ecological damage more highly than do resource managers and regulators. Ecologists and environmental activists are not, however, the people who make the decisions about undertaking insecticide spray programs to manage populations of budworm or other pests.
After 1986, fenitrothion was the only synthetic insecticide used against budworm. In 1993, a risk assessment of this use (Pauli et al., 1993) concluded that many of its ecotoxicological damages are significant: “The weight of evidence accumulated with respect to the identified and potential negative impacts caused by the forestry use of fenitrothion on non-target fauna and their potential ecological implications, supports the conclusion that the large-scale spraying of fenitrothion for forest pest control, as currently practised operationally, is environmentally unacceptable.”
Partly because of the strong conclusions of that risk assessment, the registration of fenitrothion for use in budworm spray programs in Canada was withdrawn in 1995. This action left B.t., a bacterial insecticide that causes little non-target damage, as the major insecticide available for spraying against this forest pest.
Alternatives to Insecticide
In addition to ecotoxicity caused by any insecticide, spray programs against budworm have other drawbacks from a forest-management perspective. Within limits, treating infested stands with insecticide maintains fir-spruce forest “alive and green” and therefore available as a resource for the economically important forest industry. However, spraying also maintains good habitat for budworm, and so may prolong its outbreaks. Agencies that spray insecticide can become “locked” into this pest-management tactic and must continue to spray if the forest resource is to be maintained in an economically viable condition. In the absence of an alternative control practice, spraying may be perceived to be the best available short-term tactic. Clearly, however, spray programs over extensive areas are not desirable.
An alternative to spraying insecticides may be the use of silvicultural practices that are aimed at reducing the vulnerability of stands to budworm infestation. For example, relatively tolerant species such as black spruce might be planted extensively. Alternatively, the landscape could be structured so that the total area of mature but vulnerable forest is kept small. If the landscape mosaic was dominated by less vulnerable stands that could be harvested by industry at about the rate at which the trees mature, a smaller area would be vulnerable to budworm infestation. It must be recognized, however, that such actions would represent a huge intensification of forest management and would cause enormous changes in the ecological character of the landscape. Little is known about the long-term economic viability or ecological consequences of such changes in forest-management strategy.
Further research into alternative methods of budworm control may yield novel methods that are effective. Some promise has been demonstrated by the release of large numbers of tiny Trichogramma wasps that are parasites of budworm, by the use of synthetic hormones that disrupt moulting or mating of the budworm, and by several other innovative biotechnologies. So far, however, none of these methods have proven sufficiently effective to be used in operational programs to reduce an irruption of budworm.
If regulators decide that the resource damage caused by spruce budworm must be controlled, it is desirable to utilize the least damaging, but still effective, options. At present, effective control appears to require insecticide, with the only available one being B.t. The use of synthetic organic insecticides in budworm spray programs, with their attendant ecotoxicological damage, appears to be history.
Herbicides in Forestry
The most common use of herbicide in forestry is to keep weeds from competing with young conifers, allowing them to grow more rapidly so that harvests can be more frequent (Newton and Knight, 1981; Freedman, 1995). The forestry use of herbicide accounts for less than 2% of the total use of these chemicals in Canada. However, forestry use affects the habitat of many native species, whereas this is not the case in agriculture and horticulture. In 2013, about 119-thousand ha of forestland were treated with herbicide in Canada (Thompson and Pitt, 2012).
As in agriculture and horticulture, there are alternatives to the use of herbicide in forestry. These options include the crushing of competing vegetation with large machines, manual cutting of weeds with brush saws, prescribed burning, and even using sheep to selectively browse weedy plants. These alternatives can provide a degree of weed control, but foresters generally regard them as more expensive and less effective than herbicide use.
Image 22.4. This is a four-year-old clear-cut of a coniferous forest in Nova Scotia. Almost all of the vegetation is considered (by foresters) to be “weeds” that compete with desired conifer seedlings for space, water, and nutrients. The objective of a herbicide treatment is to reduce the abundance of weeds, so as to allow the conifers to grow more quickly. Source: B. Freedman.
As with any pesticide, the successful use of herbicide for a management purpose requires the selection of an appropriate chemical and its proper application. If the right choices are not made, the weeds will not be adequately controlled, and the conifer crop may be injured. In addition, the suppression of “weeds” affects the useful ecological services that they provide, such as helping to control erosion and reducing losses of nutrients by leaching. Using herbicide also reduces employment opportunities available in manual weed-control programs. For these and other reasons, including the fears that many people have about the potential toxicological risks of pesticides in the environment, the use of herbicide (and insecticide) in forestry has been very controversial.
Weeds in Forestry
Any disturbance of a forest is followed by vigorous regeneration involving many species of plants that compete for space, nutrients, and moisture. This is true whether the disturbance is caused by a natural fire, insect attack, or clear-cutting. During the first 10 to 15 years of succession, the plant community is dominated by many plants other than the conifers that are desired by the forest industry. This can be illustrated by examining vegetation data for young clear-cuts in Nova Scotia, where conifers contributed only 4-9% of the plant cover (Figure 22.4). Other species that are not economically desirable (from the forestry perspective) are much more abundant – these “weeds” include ferns, monocotyledonous plants such as sedges and grasses, dicotyledonous herbs such as asters and goldenrods, low shrubs such as raspberries and blackberries, and taller shrubs such as birches, maples, and cherries. The dominance of the site by “undesirable” plants inhibits the growth of commercially desired conifers, and provides an economic justification for a weed-management treatment.
Figure 22.4. Dominant Plants in Disturbed Forest. The vegetation was surveyed in four clear-cuts of conifer forest in Nova Scotia that were 4-6 years old. The data represent the range of average plant cover among the four sites, expressed as the percentage of ground that is obscured by foliage. Because of overlap, cover values can exceed 100%. Source: Data from Freedman (1995).
The effects of competing plants on conifer productivity are illustrated by a study of a site in New Brunswick that had been treated with herbicides 28 years previously (MacLean and Morgan, 1983). Prior to the herbicide treatment, the vigorously growing vegetation had been dominated by angiosperm shrubs that formed a dense, 2-m-tall canopy that overtopped the shorter conifers. The herbicide spray had released the conifers from some of the stresses of competition. The study consequently found, 28 years after the herbicide treatment, that the biomass of balsam fir on the sprayed plots was about three-times larger than on an adjacent unsprayed plot. From the forestry perspective, this means that the herbicide treatment allowed a conifer-dominated stand to develop more quickly, shortening the time to the next harvest.
Toxicological and Ecological Effects
The silvicultural objective of herbicide spraying is to manage vegetation by changing its character. A herbicide treatment in forestry reduces the abundance of competing vegetation, but it rapidly recovers. In essence, a herbicide treatment returns the post-harvest regeneration (usually post-clear-cutting) to an earlier successional stage, while for several years releasing small conifer plants from some effects of competition. The changes in vegetation are illustrated by a study in Nova Scotia, in which a substantial recovery occurred within only one growing season after a herbicide treatment (Figure 22.5). The recovery involved species whose seeds colonize sprayed sites, as well as plants that were not killed by the herbicide. This study found that no species were eliminated from sprayed clear-cuts, although large differences occurred in their relative abundance between sprayed and reference (unsprayed) plots. This is because species vary in susceptibility to herbicides and in their ability to recover from a disturbance.
Figure 22.5. Recovery of Vegetation after a Herbicide Treatment. Regenerating clear-cuts were treated with the herbicide glyphosate at two sites in Nova Scotia. The reference plots were not sprayed and illustrate the normal recovery of vegetation after clear-cutting. The sprayed plots were sampled for one year prior to the herbicide treatment, and the post-spray recovery was then monitored for several years. The data are percentage plant cover at the end of the summer. Source: Data from Freedman et al. (1993).
Compared with many insecticides, herbicides used in forestry (such as 2,4,5-T, 2,4-D, and glyphosate) are not very toxic to animals (see Table 22.2). At exposures encountered by animals during typical forestry uses, the direct toxicological risks are probably unimportant. This is particularly true of glyphosate, the most commonly used herbicide.
Glyphosate is extremely toxic to most plants, acting by blocking the synthesis of several essential amino acids. All plants and some microorganisms use this metabolic pathway, but animals obtain these amino acids in their food. Consequently, glyphosate is non-toxic to animals (see Tables 15.2 and 22.2).
Nevertheless, glyphosate causes large changes to occur in habitat because it affects the productivity and biomass of plants. Birds and other animals can be affected by a decreased availability of berries and other plant foods. In addition, the reduced foliage biomass on sprayed areas sustains a lower abundance of insects and spiders, which are important foods for most birds. These are indirect ecotoxicological effects of herbicide spraying, and they affect birds and other wildlife even if they are not directly poisoned.
Image 22.5. A helicopter releases a silvicultural application of the herbicide glyphosate to a clear-cut in Nova Scotia. Source: B. Freedman.
A study in Nova Scotia found only small changes in the abundance of birds that were breeding on clear-cuts treated with glyphosate (Table 22.7). Although the avian population decreased between the pre-spray and first post-spray years, this also occurred on the reference plot, suggesting it was caused by a factor unrelated to the herbicide treatment, such as bad weather. In the second year after spraying, the abundance of birds on the sprayed plots was similar to the first post-spray year, while on the unsprayed plot it increased to about the pre-spray value.
Table 22.7. Populations of Breeding Birds on Herbicide-Treated Clear-Cuts. Average data are presented for four sprayed plots and one unsprayed reference plot from a study in Nova Scotia. The sprayed plots were treated with glyphosate. The data are pairs of breeding birds per square kilometre, surveyed for one year before the herbicide treatment (year 0), and then for four post-spray years. Only abundant species are listed here. Source: Data from MacKinnon and Freedman (1993).
The most common species were white-throated sparrow and common yellowthroat, which had a decreased abundance on both the sprayed and reference plots up to the second year after spraying, and then recovered by the fourth post-spray year. On the reference plot, song sparrow and Lincoln’s sparrow declined during the course of the study, whereas on the sprayed plots they were most abundant in the second and fourth years after spraying. The reference plot became colonized by some new species, including black-and-white warbler, red-eyed vireo, ruby-throated hummingbird, and palm warbler. These species did not invade the sprayed plots because the herbicide treatment caused the habitat to revert to a younger stage that was less favourable to these birds.
Most studies of the effects of herbicides on deer and moose have examined the availability of their food. Broad-leaved shrubs are a preferred food (known as browse) for species of deer, but they are also important weeds in forestry and are a target of herbicide treatments. The quantity of browse, although initially reduced by herbicide spraying, is often increased in the medium-term through the regeneration of shrubs. For example, studies in Maine found that several years after spraying, the availability of browse was greater on treated clear-cuts, partly because the height of the shrub canopy was lower, which gave white-tailed deer easier access to this food (Newton et al., 1989). This is not always the case, however, and some studies have shown that herbicide spraying may decrease the habitat quality for deer.
Overall, field research suggests that herbicide use in forestry has relatively small effects on birds and other wildlife that utilize clear-cuts as habitat. Other disturbances associated with forestry cause much larger effects on wildlife, particularly clear-cutting and the conversion of the natural forest into a plantation (see Chapter 23).
The hazards to people from herbicide use in forestry have also been scrutinized. The concerns include occupational risks for people who are engaged in spraying or are working in recently sprayed areas, as well as risks for the general population. These issues are highly controversial. Much of the concern has focused on the phenoxy herbicides 2,4,5-T and 2,4-D, partly because 2,4,5-T may contain a trace contamination of TCDD, a toxic dioxin. However, the use of other herbicides, such as glyphosate, is also controversial.
It is somewhat reassuring to know that many scientists believe that herbicides can be used safely in forestry (and in agriculture and horticulture), provided the instructions for their use are followed carefully. Many scientists also believe that herbicides do not cause undue risks to sprayers or people living near the treated areas. These are among the reasons why governments have registered these pesticides for uses that are economically beneficial by allowing greater productivity of both agricultural and forest crops. It must be remembered, however, that scientists have not achieved a full consensus on these issues. Partly for this reason, the use of pesticides in forestry and for other purposes continues to be highly controversial.
Integrated Pest Management
Pesticides are commonly used in agriculture, horticulture, and forestry. It is clear from this fact that most politicians, bureaucrats, and resource managers – and many scientists – have decided that the environmental “costs” associated with the use of pesticides are “acceptable” in view of economic benefits that are achieved. It is debatable, however, whether reliance on pesticide use is desirable over the long term. This is particularly true for those pesticides that are toxic to a broad spectrum of organisms. Most people would prefer that less reliance be placed on such non-specific methods of pest control.
A much preferable approach is known as integrated pest management (IPM), which employs an array of complementary tactics toward achieving pest control, with the aim of there being fewer environmental and health risks. Elements of an IPM system can include the following:
- use of natural predators, parasites, and other biological agents that can help to control a pest, while causing few non-target damages
- use of crop varieties that are resistant to pests
- management of habitat to make it less suitable for pests
- careful monitoring of pest abundance, so control measures are undertaken only when necessary
- use of pesticides, but only if required as a component of an IPM strategy
A successful IPM program can greatly reduce, but not necessarily eliminate, reliance on pesticides. For example, for many years the cultivation of cotton in the southern United States relied on the intensive application of insecticides against pests such as the boll weevil. Widespread use of an IPM system to control this insect in Texas cottonfields reduced insecticide use from 8.8 million kilogram in 1964 to 1.05 million kilogram in 1976 (Bottrell and Smith, 1982). Nevertheless, insecticide use against this pest remained necessary.
Wherever possible, IPM systems utilize control methods that are as specific as possible to the pest so that non-target damage can be avoided or greatly reduced. Some of the best examples of such specific methods involve biological control (the use of a biological agent). The usefulness of biological control can be illustrated with the following successful controls of introduced agricultural pests (Freedman, 1995):
- The cottony-cushion scale (Icyera purchasi) is a sap-sucking insect that was accidentally introduced to the United States, where it became a threat to citrus agriculture. Research in its native Australia discovered that the pest was naturally controlled by certain insect predators and parasites. In 1888, two of its predators, a lady beetle and a parasitic fly, were introduced to California. This allowed almost total control over this potentially disastrous pest. Unfortunately, this biological control was disrupted when DDT and other broad-spectrum insecticides were used to deal with other orchard pests beginning in the late 1940s.
- St. John’s wort (Hypericum perforatum), a common weed, is toxic to cattle. It became a serious pest in pastures after it was introduced from Europe. In 1943, two leaf beetles that feed on this plant were released to North America, and this pest is no longer an important problem.
- The prickly pear cactus (Opuntia stricta) was imported to Australia from North America and grown as an ornamental plant and a “living fence.” It escaped and became a serious weed in rangelands. This pest was controlled by the introduction of one of its herbivores, a moth whose larvae feed on the cactus.
- Ragwort (Senecio jacobea) is a Eurasian plant that has been introduced to the Americas and Australia. It became an important weed in rangeland because it crowds out native plants and is toxic to cattle. Several of its Eurasian herbivores are now being used to control its abundance, including the cinnabar moth, ragwort flea beetle, and ragwort seed fly.
- The screw-worm fly (Callitroga hominivorax) causes damage to cattle when its larvae feed on open wounds. This pest has been controlled in some areas through the release of large numbers of male flies that were reared in laboratories and sterilized by irradiation. Because the female flies mate only once, copulation with a sterile male results in unsuccessful reproduction. If this happens to enough females, the abundance of the pest decreases to an acceptable level.
Unfortunately, biological control may not be suitable for all pest problems, and in fact it has not succeeded in most cases in which it has been attempted. The list of failures includes forest pests such as spruce budworm and gypsy moth. Researchers have investigated the potential for controlling budworm using pest-specific bacteria, viruses, and other agents of diseases, wasps that parasitize and kill larvae, and sex and developmental hormones to disrupt mating and growth. Some of the biological control methods have shown promise, but they do not yet achieve a consistent kill of budworm and are relatively expensive. For these reasons, they are not considered ready for routine use against this important pest.
The only viable alternative to the broadcast spraying of synthetic insecticides to control budworm is an insecticide based on the bacterium B.t. (examined earlier). Research into other biological controls continues, and some may yet prove successful and would allow managers to develop an effective IPM system that does not rely on broadcast spraying of insecticides, even relatively specific ones such as B.t.
At the present time, however, it appears that society will continue to rely heavily on pesticide use in intensively managed systems in agriculture and forestry. This will happen even though the intensive systems cause ecological damage (partly because this damage is not fully accounted for as an economic “cost”).
It is important that additional research is undertaken to develop viable methods of biological control and other elements of IPM systems. This is necessary if the present reliance on the pesticide treadmill is to be replaced with less-damaging methods of pest management. Such a change would deliver substantial benefits to society, because the agricultural and forestry systems that we require for sustenance could be managed on a more ecologically sustainable basis. In part, this will require that more attention be directed to the ecological damage caused by the intensive use of pesticides.
Because of this damage, it is highly desirable that non-pesticidal alternatives to pest control are discovered as quickly as possible. Until this happens, pesticide use should be reduced to the lowest levels that continue to effectively control the pests. Some environmentalists have argued that pesticide use in North America is much greater than necessary and that it could be decreased without causing a significant adverse effect on crop yields. In fact, the European Union has already passed legislation requiring that agricultural pesticide use be reduced by half, and many pesticides have been banned. Serious consideration should also be given to such actions in Canada.
Canadian Focus 22.2 Municipal Bans on Pesticide Use Most Canadian municipalities have banned the routine use of pesticides to manage lawns and gardens. This was done in response to concerns about the exposure of humans and pets to pesticide residues in the urban environment. Because most pesticide use in horticulture is essentially for cosmetic purposes, and alternative pest-management practices are available, it is believed that no substantial economic detriment would be suffered from the pesticide bans.
In 1991, the town of Hudson, Quebec, passed the first bylaw to regulate horticultural pesticide use. Opponents of that law included lawn-care companies and pesticide manufacturers, and they were successful in having it struck down by the Quebec Court of Appeal. In 2000, however, the Supreme Court of Canada reversed that decision and made it legal for municipalities to regulate pesticide use on lands within their jurisdiction. Since then, many additional municipalities have banned the use of pesticides for cosmetic horticultural purposes.
In 2000, the Halifax Regional Municipality enacted a bylaw that prohibits the use of horticultural pesticides within 50 m of any park or playground, daycare centre, senior-citizen residence, public school, university, church, or hospital. In the remaining places where pesticides could still be used, homeowners wanting to use them must apply for a permit, and if it is granted, they must post a prominent sign for one day before and four days after the application. In a typical year, several hundred permit applications are received, most of which are for use against chinch bugs (Blissus leucopterus) in lawns. Almost all of the applications are made by lawn-care companies on behalf of their clients, and about half are approved. The Halifax bylaw is controversial, and it has been resisted by lawn-care companies, some gardeners, and other interests. Paradoxically, a weakness in the Halifax bylaw is that it does not ban the sale of pesticides – only most of their uses. Because pesticides can be so easily obtained, compliance with the bylaw is substantially voluntary, and no one has ever been charged with violating it. In spite of this weakness, the bylaw has been widely applauded as a step forward in the broader environmental sense.
In 2003, the council of Toronto, the largest city in Canada, passed a pesticide bylaw and in so doing greatly bolstered country-wide efforts across our country. In 2009, Ontario enacted a province-wide ban on the sale and use of pesticides for cosmetic purposes. These and ongoing actions to restrict the unnecessary use of pesticides have resulted from concerted actions of citizens and appropriate governmental responses, and they are an environmental “success story”.
Conclusions
Pesticides are a wide range of substances that are used to gain an advantage over species that cause diseases or are pests in agriculture, forestry, or horticulture. However, the use of many pesticides carries risks of causing damage to human health or the environment. Pesticides have become an integrated component of most of the intensive systems by which foods and other crops are grown, and there are not yet good replacements for all of their uses. For this reason, the use of pesticides will continue into the foreseeable future. Nevertheless, it is important that more research be done to find effective ways of reducing the dependence on pesticides, especially on integrated pest-management systems and on means of biological control. In the meantime, it is important that pesticide use be reduced to the lowest amounts possible and that the most damaging chemicals are withdrawn from legal use.
Questions for Review
- Classify pesticides according to their intended targets and also by their major chemical groups.
- What is a “pest”? Why do people consider it necessary to manage their abundance in agriculture, horticulture, forestry, and public health?
- What characteristics of organochlorines have caused them to become global contaminants? Why do they pose special toxicological risks to top predators?
- In view of the fact that spruce budworm is a native insect, why is the damage it causes to conifer forest considered to be a problem?
Questions for Discussion
- Identify and compare the benefits and environmental risks associated with pesticide use in one of agriculture, horticulture, or forestry.
- Are there effective alternatives to the continued use of pesticides? Consider the roles of integrated pest management, biological controls, and other options.
- For many common uses of pesticides, there are alternative ways of managing the targeted pest. For example, weeds in a lawn can be controlled by digging them out, rather than by using a herbicide. Pest rodents could be trapped instead of being killed with a rodenticide. What do you think should be the key considerations when deciding whether to use pesticides or alternative means of control?
- Some people believe that the use of pesticides should be allowed only in extreme cases, for example, to save human lives or to prevent a food catastrophe. Most pesticide use, however, is more routine than this. What do you think about this issue? Should it be made more difficult for people to use pesticides? Or should farmers and other potential users have freedom to make their own choices about pesticide use?
Exploring Issues
- A large corporation is seeking permission from the government to market a new insecticide in Canada. You are a wildlife biologist and have been asked to recommend studies to identify whether unacceptable damages would be caused to animals, plants, or ecosystems by the new insecticide. How would you design such a study? What specific questions would you want to answer?
- You are an environmental scientist and have been asked to provide expert advice about a proposed new municipal bylaw regulating the cosmetic use of pesticides in horticulture. The bylaw itself could be similar to the one used in Halifax, as described in Canadian Focus 22.2. From the environmental perspective, what would you consider to be the benefits and problems with the proposed bylaw?
References Cited and Further Reading
Agriculture Canada. 1993. Registration Status of Fenitrothion Insecticide. Discussion Report 093-01, Food Production and Inspection Branch, Agriculture Canada, Ottawa, ON.
Armstrong, J.A. 1985. Spruce budworm control program in eastern Canada. Pp. 384-385 In: Advances in Spruce Budworms Research. Canadian Forestry Service, Ottawa, ON.
Bishop, C. and D.V. Weseloh. 1990. Contaminants in Herring Gull Eggs from the Great Lakes. SOE Fact Sheet 90-2, Environment Canada, Ottawa.
Blais, J.R. 1985. The ecology of the eastern spruce budworm. Pp. 49-59 in: Recent Advances in Spruce Budworms Research. Canadian Forestry Service, Ottawa, ON.
Bottrell, D.G. and R.F. Smith. 1982. Integrated pest management. Environmental Science and Technology, 16: 282A-288A.
Briggs, S.A. 1992. Basic Guide to Pesticides: Their Characteristics and Hazards. Taylor & Francis, Washington, DC.
Busby, D.G., L.M. White, P.A. Pearce, and P. Mineau. 1989. Fenitrothion effects on forest songbirds: A critical new look. Pp. 43-108 in: Environmental Effects of Fenitrothion Use in Forestry. Environment Canada, Dartmouth, NS.
Canadian Forest Service. 2014. The State of Canada’s Forests. Annual Report 2014. Natural Resources Canada, Canadian Forest Service, Ottawa, ON.
Carson, R. 1962. Silent Spring. Houghton-Mifflin, Boston, MA.
Cooper, K. 1991. Effects of pesticides on wildlife. Pp. 463-496 in: Handbook of Pesticide Toxicology. Vol. 1, General Principles. (W.C. Hayes and E.R. Laws, eds.). Academic Press, San Diego, CA.
Crawford, H.S., R.W. Titterington, and D.T. Jennings. 1983. Bird predation and spruce budworm populations. Journal of Forestry, 81: 433-435.
Edwards, C.A. 1975. Persistent Pesticides in the Environment. CRC Press, Cleveland, OH.
Ennis, T. and E.T.N. Caldwell. 1991. Spruce budworm, chemical and biological control. Pp. 621-641 in: Tortricid Pests, Their Biology, Natural Enemies, and Control. (L.P.S. van der Geest and H.H. Evenhuis, eds.). Elsevier, Amsterdam.
Environment Canada. 1993. Toxic Contaminants in the Environment: Persistent Organochlorines. Ottawa: State of the Environment Reporting. Environment Canada. 1996. The State of Canada’s Environment, 1996. Government of Canada, Ottawa, ON.
Environment Canada. 2001. Assessment Report; Nonylphenol and Its Ethoxylates. Environment Canada. Ottawa, ON.
Environmental Protection Agency (EPA). 2003. The Great Lakes; An Environmental Atlas and Resource Book. U.S. Environmental Protection Agency, Washington, DC.
Environmental Protection Agency (EPA). 2008. “Inert or “Other” Ingredients in Pesticide Products. U.S. Environmental Protection Agency, Washington, DC.
Freedman, B. 1995. Environmental Ecology. 2nd ed. Academic Press, San Diego, CA.
Freedman, B., R. Morash, and D. MacKinnon. 1993. Short-term changes in vegetation after the silvicultural spraying of glyphosate herbicide onto regenerating clear-cuts in Nova Scotia, Canada. Canadian Journal of Forest Research, 23: 2300-2311.
Gage, S.H. and C.A. Miller. 1978. A Long-Term Bird Census in Spruce Budworm-Prone Balsam Fir Habitats in Northwestern New Brunswick. Information Report M-X-84, Maritimes Forest Research Centre, Fredericton, NB.
Gianessi, L.P. and S. Sankula. 2003. The Value of Herbicides in U.S. Crop Production. National Centre for Food and Agricultural Policy, Washington, DC.
Grossbard, E. and D. Atkinson (eds.). 1985. The Herbicide Glyphosate. Butterworths, London, UK.
Grube, A., D. Donaldson, T. Kiely, and L. Wu. 2007. Pesticides Industry Sales and Usage, 2006 and 2007, Market Estimates. U.S. Environmental Protection Agency, Washington, DC. https://www.epa.gov/sites/production/files/2015-10/documents/market_estimates2007.pdf
Hallmann, C.A., R.P.B. Foppen, C.A.M. van Turnhout, H. de Kroon, and E. Jongejans. 2014. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature, 511: 341-343.
Hayes, W.J. 1991. Introduction. Pp. 1-37 in: Handbook of Pesticide Toxicology. Vol. 1, General Principles. (W.C. Hayes and E.R. Laws, eds.). Academic Press, San Diego, CA.
Jeschke, P., R. Nauen, M. Schindler, and A. Elbert, 2011. Overview of the status and global strategy for neonicotinoids. Journal of Agricultural and Food Chemistry, 59: 2897-2908.
Kamrin, M.A. (ed.). 1997. Pesticide Profiles: Toxicity, Environmental Impact, and Fate. Lewis Publishers, Boca Raton, FL.
Kettela, E. 1983. A Cartographic History of Spruce Budworm Defoliation from 1967 to 1981 in Eastern North America. Information Report DPC-X-14, Maritimes Forest Research Centre, Canadian Forestry Service, Fredericton, NB.
Kingsbury, P.D. and B.B. McLeod. 1981. Fenitrothion and Forest Avifauna Studies. Report FPM-X-43, Forest Pest Management Institute, Canadian Forestry Service, Sault Ste. Marie, ON.
Krieger, R. 2001. Handbook of Pesticide Toxicology. 2nd ed. Academic Press, San Diego, CA.
Landis, J.N., J.E. Sanchez, G.W. Bird, C.E. Edson, R. Isaacs, R.H. Lehnert, A.M.C. Shilder and S.M. Swinton (eds). 2002. Fruit Crop Ecology and Management. Publication E2759, Integrated Pest Management, Michigan State University Extension, Lansing, MI.
MacKay, D., W.Y. Shiu, and K.-C. Ma. 1997. Illustrated Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals: Pesticide Chemicals. Lewis Publishers, Boca Raton, FL.
Mackinnon, D. and B. Freedman. 1993. Effects of silvicultural use of the herbicide glyphosate on breeding birds of regenerating clear-cuts in Nova Scotia, Canada. Journal of Applied Ecology, 30: 395-406.
MacLean, D.A. 1988. Effects of spruce budworm outbreaks on vegetation, structure, and succession of balsam fir forests on Cape Breton Island, Canada. Pp. 253-261 in: Plant Form and Vegetation Structure. SPB Academic, The Hague, The Netherlands.
MacLean, D.A. 1990. Impact of forest pests and fire on stand growth and timber yield: implications for forest management planning. Canadian Journal of Forest Research, 20: 391-404.
MacLean, D.A. and M.G. Morgan. 1983. Long-term growth and yield response of young fir to manual and chemical release from shrub competition. Forestry Chronicle, 59: 177-183.
McEwen, F.L. and G.R. Stephenson. 1979. The Use and Significance of Pesticides in the Environment. Wiley, New York.
Millikin, R.L. 1990. Effects of fenitrothion on the arthropod food of tree-foraging forest songbirds. Canadian Journal of Zoology, 68: 2235-2242.
Milne, G.W.A. 1994. CRC Handbook of Pesticides. CRC Press, Boca Raton, FL.
Mineau, P. 1993. The Hazard of Carbofuran to Birds and Other Vertebrate Wildlife. Technical Report No. 177, Wildlife Toxicology Section, Canadian Wildlife Service, Ottawa, ON.
Mineau, P. 1999. Pesticides and Wild Birds. Ottawa, ON: Canadian Wildlife Service. http://webarchive.bac-lac.gc.ca:8080/wayback/20141223193647/http://www.hww.ca/en/issues-and-topics/pesticides-and-wild-birds.html
Mineau, P. and C. Palmer. 2013. The Impact of the Nation’s Most Widely Used Insecticides on Birds. Neonicotinoid Insecticides and Birds, American Bird Conservancy. http://www.abcbirds.org/abcprograms/policy/toxins/Neonic_FINAL.pdf
Moriarty, F. 1999. Ecotoxicology: The Study of Pollutants in Ecosystems. 3rd ed. Academic Press, London, UK.
Morris, R.F., W.F. Cheshire, C.A. Miller, and D.G. Mott. 1958. The numerical response of avian and mammalian predators during a gradation of the spruce budworm. Ecology, 39: 487-494.
National Research Council (NRC). 1986. Pesticide Resistance. NRC, National Academy Press, Washington, DC.
National Research Council (NRC). 1996. Ecologically Based Pest Management: New Solutions for a New Century. NRC, National Academy Press, Washington, DC.
Newton, M. and F.B. Knight. 1981. Handbook of Weed and Insect Control Chemicals for Forest Resource Managers. Timber Press, Beaverton, OR.
Newton, M., E.C. Cole, R.A. Lautenschlager, D.E. White, and M.L. McCormack. 1989. Browse availability after conifer release in Maine’s spruce-fir forests. Journal of Wildlife Management, 53: 643-649.
Norheim, G., L. Somme, and G. Holt. 1982. Mercury and persistent chlorinated hydrocarbons in Antarctic birds. Environmental Pollution, (Series A), 28: 233-240.
Ostaff, D.P. 1985. Quantifying effects of spruce budworm damage in eastern Canada. Pp. 247-248 in: Recent Advances in Spruce Budworms Research. Canadian Forestry Service, Ottawa, ON.
Ostaff, D.P. and D.A. MacLean. 1989. Spruce budworm populations, defoliation, and changes in stand condition during an uncontrolled spruce budworm outbreak on Cape Breton Island, Nova Scotia. Canadian Journal of Forest Research, 19: 1077-1086.
Pauli, B.D., S.B. Holmes, R.J. Sebastien, and G.P. Rawn. 1993. Fenitrothion Risk Assessment. Technical Report Series No. 165, Canadian Wildlife Service, Ottawa, ON.
Peakall, D.B. 1990. Prospects for the peregrine falcon, Falco peregrinus, in the nineties. Canadian Field-Naturalist, 104: 168-173.
Pearce, P.A. and D.B. Peakall. 1977. The impact of fenitrothion on bird populations in New Brunswick. Pp. 299-305 in: NRCC 16073, National Research Council of Canada, Ottawa, ON.
Pesticide News. 2003. Editorial No. 60.
Pimentel, D. (ed.). 1997. Techniques for Reducing Pesticide Use: Economic and Environmental Benefits. John Wiley & Sonsm New York, NY.
Pimentel, D. and H. Lehman (eds.). 1992. The Pesticide Question: Environment, Economics, and Ethics. Chapman & Hall, New York, NY.
Pimentel, D., H. Acquay, M. Biltonen, P. Rice, M. Silva, J. Nelson, V. Lipner, S. Giordano, A. Horowitz, and M. D’Amare. 1992. Environmental and economic costs of pesticide use. Bioscience, 42: 750-760.
Pimentel, D., L. McLaughlin, A. Zepp, B. Lakitan, T. Kraus, P. Kleinman, F. Vancini, W.J. Roach, E. Graap, W.S. Keeton, and S. Selig. 1991. Environmental and economic effects of reducing pesticide use. Bioscience, 41: 402-409.
Ryckman, D.P., D.V. Weseloh, and C.A. Bishop. 1997. Contaminants in Herring Gull Eggs from the Great Lakes: 25 Years of Monitoring Levels and Effects. Environment Canada, Ottawa, ON. http://webarchive.bac-lac.gc.ca:8080/wayback/20060117194755/http://www.on.ec.gc.ca/wildlife/factsheets/pdf/fs_herring_gulls_e.pdf
Rozencranz, A. 1988. Bhopal, transnational corporations, and hazardous technologies. Ambio, 17: 336-341.
Sassman, J., R. Pienta, M. Jacobs, and J. Cioffi. 1984. Pesticide Background Statements. Vol. 1, Herbicides. USDA Forest Service, Washington, DC. Stenersen, J. 2004. Chemical Pesticides: Mode of Action and Toxicology. CRC Press, Boca Raton, FL.
Sullivan, T.P. and D.S. Sullivan. 2003. Vegetation management and ecosystem disturbance: Impact of glyphosate herbicide on plant and animal diversity in terrestrial systems. Environmental Reviews: 11: 37-59.
Thompson, D. and D. Pitt. 2012. Forest Herbicide Research. Natural Resources Canada, Canadian Forest Service, Great Lakes Forestry Centre, Sault Ste. Marie, ON.
van der Sluijs, J.P., V. Amaral-Rogers, L.P. Belzunces, M.F.I.J.B. van Lexmond, J-M. Bonmatin, M. Chagnon, C.A. Downs, L. Furlan, D.W. Gibbons, C. Giorio, V. Girolami, D. Goulson, D.P. Kreutzweiser, C. Krupke, M. Liess, E. Long, M. McField, P. Mineau, E.A.D. Mitchell, C.A. Morrissey, D.A. Noome, L. Pisa1, J. Settele, N. Simon-Delso, J.D. Stark, A. Tapparo, H. Van Dyck, J. van Praagh, P.R. Whitehorn, and M. Wiemers. 2014. Conclusions of the Worldwide Integrated Assessment on the risks of neonicotinoids and fipronil to biodiversity and ecosystem functioning. Environmental Science and Pollution Research, (online). http://link.springer.com/article/10.1007/s11356-014-3229-5/fulltext.html
Varty, I.W. 1975. Side effects of pest control projects on terrestrial arthropods other than the target species. Pp. 266-275 in: Aerial Control of Forest Insects in Canada. (M.L. Prebble, ed.). Department of the Environment, Ottawa, ON.
Williams, G.M., R. Kroes, and I.C. Munro. 2000. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient glyphosate for humans. Regulatory Toxicology and Pharmacology, 31: 117-165.
Winston, M.L. 1997. Nature Wars: People vs. Pests. Harvard University Press, Cambridge, MA.
Wurster, D.H., C.F. Wurster, and W.N. Strickland. 1965. Bird mortality following DDT spray for Dutch elm disease. Ecology, 46: 488-499.